Mental Health 3
Session: Mental Health 3
081 - Less is More: Strategies for Shared Decision Making and Safe Psychiatric Medications for Children with Behavioral Problems
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 81.5825
Stephanie C. Acquilano, Geisel School of Medicine at Dartmouth, Lebanon, NH, United States; Hannah B Leavitt, Geisel School of Medicine at Dartmouth, Georgetown, TX, United States; Jennifer L McLaren, Dartmouth Health, Lebanon, NH, United States; Erin R Barnett, Dartmouth Health, Lebanon, NH, United States; Stephanie Bizzari, Geisel School of Medicine at Dartmouth, Auburn, NY, United States; Anthony A. Garami, Robert Larner, M.D., College of Medicine at the University of Vermont, Plattsburgh, NY, NY, United States; Ben Fehnert, Fora Health Ltd, Amsterdam, Noord-Holland, Netherlands; James King, Fora Health, London, England, United Kingdom; Glyn Elwyn, Geisel School of Medicine at Dartmouth, Norwich, VT, United States
.jpg)
Stephanie C. Acquilano, MA (she/her/hers)
Research Project Director
Geisel School of Medicine at Dartmouth
Lebanon, New Hampshire, United States
Presenting Author(s)
Background: The number of children receiving antipsychotic medications or polypharmacy has increased sharply in recent years. Parents of children with behavioral issues may not be fully engaged in medication decisions or aware of risks. Clinicians are concerned about medication risks but may be hesitant to make changes without structured guidance. We developed the AWARE intervention to support: (a) shared decisions using a conversation aid (Figure 1) and mobile app, and (b) safe reduction of unnecessary medications using a structured protocol (Figure 2).
Objective: Our main objective was to evaluate the feasibility of AWARE to engage patients, families and prescribers in shared decision making and the safe reduction of unnecessary medications.
Design/Methods: We studied AWARE at 2 community mental health centers with 5 clinical prescribers and 31 parent-child dyads. Eligible children were aged 6-17 and prescribed polypharmacy (3 or more psychiatric medications) or an antipsychotic off-label. Dyads and prescribers used the conversation aid and mobile app to review the child’s medications and make a shared decision about whether to try to reduce the dose of at least 1 medication. We assessed feasibility based on AWARE participation over 18 weeks, outcomes of attempted dose reductions, and qualitative interviews, analyzed thematically and conducted with all 5 prescribers and a subset of 10 parents. We assessed safety in terms of types and rates of negative medication reactions, whether these reactions influenced dose reductions, and feedback from qualitative interviews.
Results: Most participants were White and non-Hispanic, 85% of parents were female and 63% of children were male (Table 1). Of 31 dyads enrolled, 29 (94%) received the AWARE intervention; 13 (45%) chose to reduce a medication, 12 (41%) chose to maintain medications, and 4 (14%) were undecided. Of the 13 who began dose reductions, 7 discontinued the medication, 3 partially reduced and 3 were unable to reduce. Five of the 7 who discontinued a medication chose a second medication to reduce. There were no reported behavioral escalations; 8 children had minor reactions to medication changes but only 2 stopped dose reductions as a result. Twenty-two parents activated the mobile app, 18 used the treatment review section and 6 used the resources section. In interviews, parents reported feeling more informed and involved in decisions; prescribers found AWARE helpful and safe.
Conclusion(s): AWARE addresses an important clinical need in children’s behavioral health, appears to be feasible and safe, and seems valued by parents and prescribers.
Figure 1. Patient-Facing Conversation Aid of Treatment Options for Children with Behavioral Problems
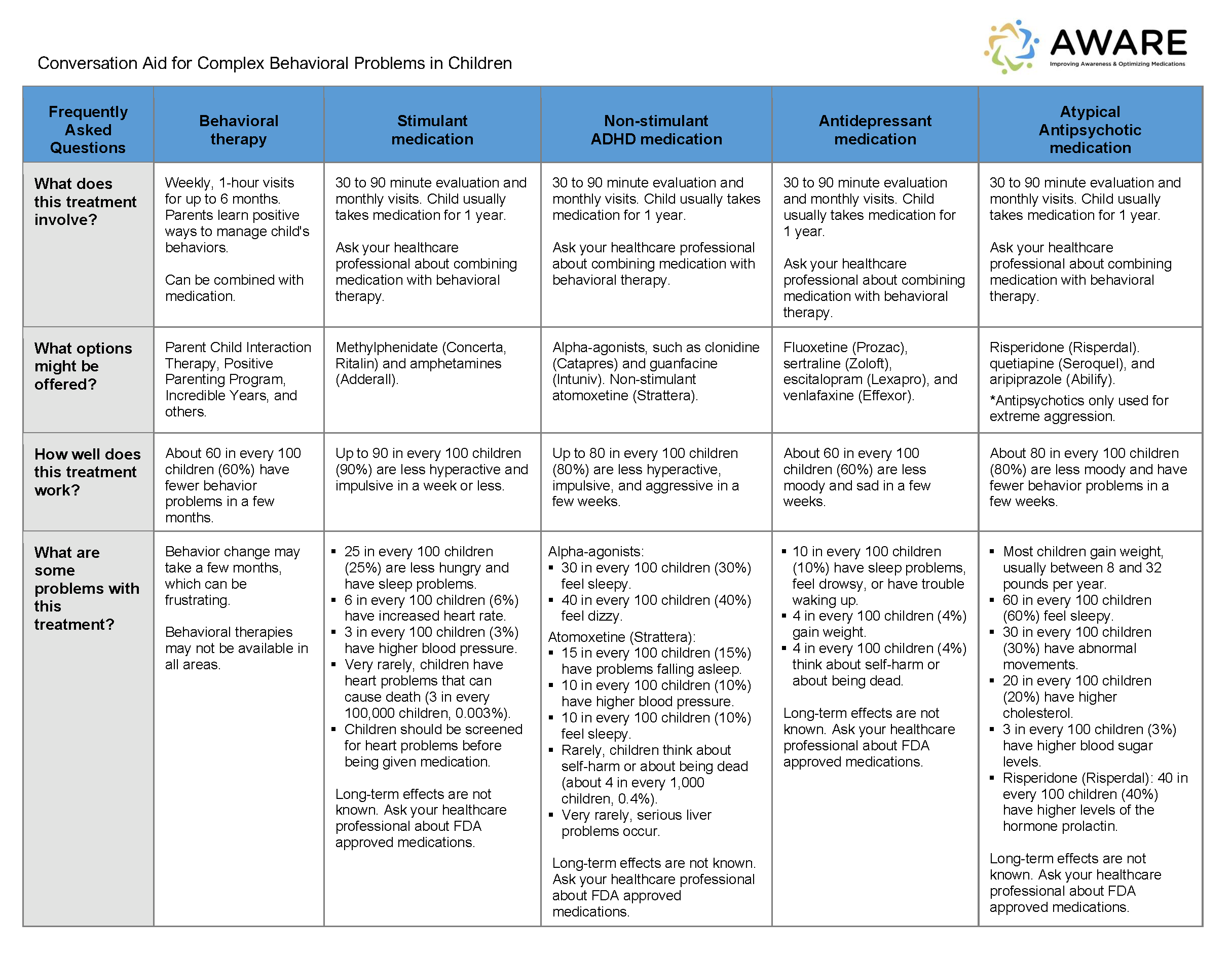
Figure 2. Detailed Guidance Table Excerpted from Structured Protocol on Safe Medication Reduction and Discontinuation
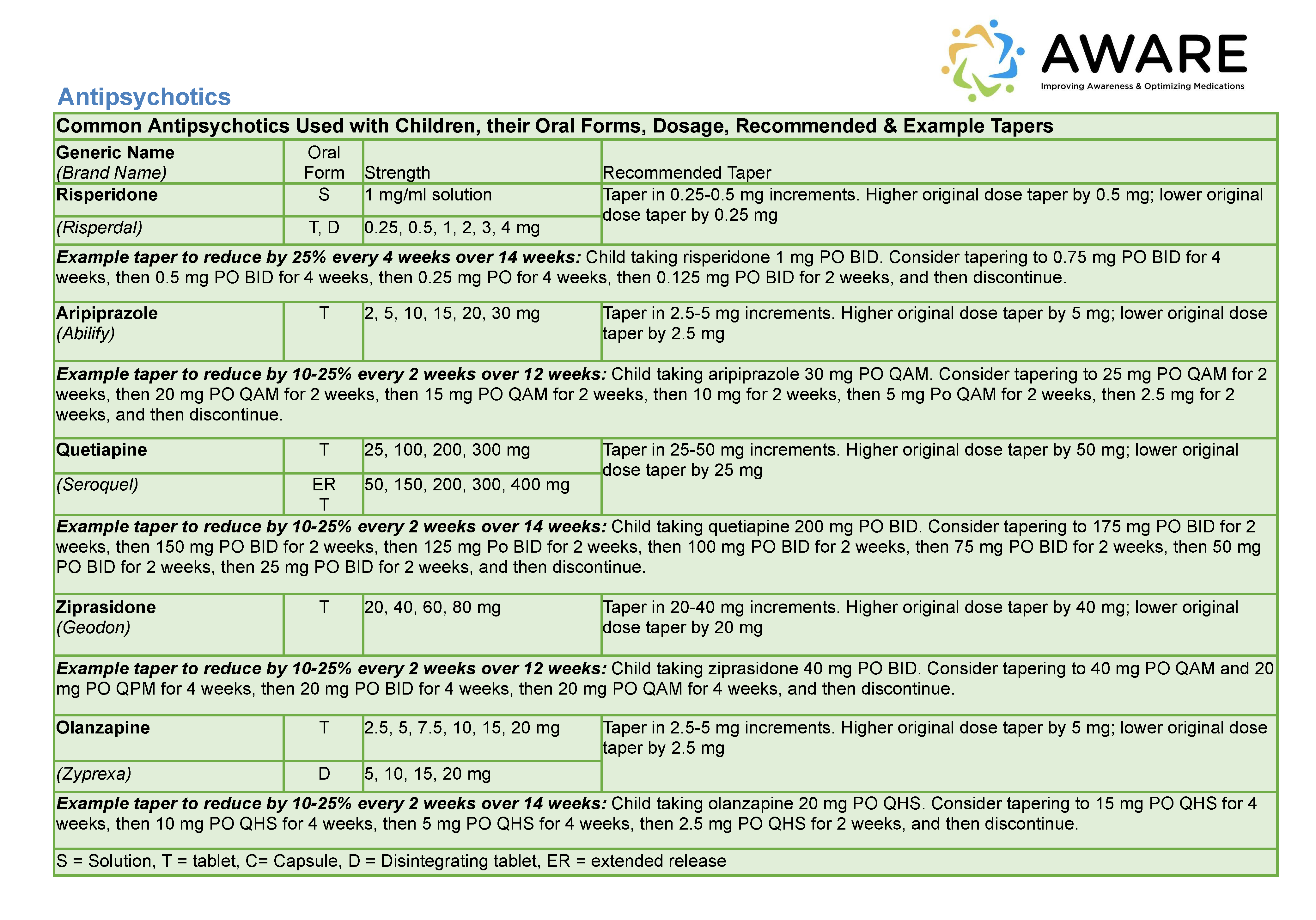
Table 1. Characteristics of Parent and Child Participants at Baseline (n = 27)
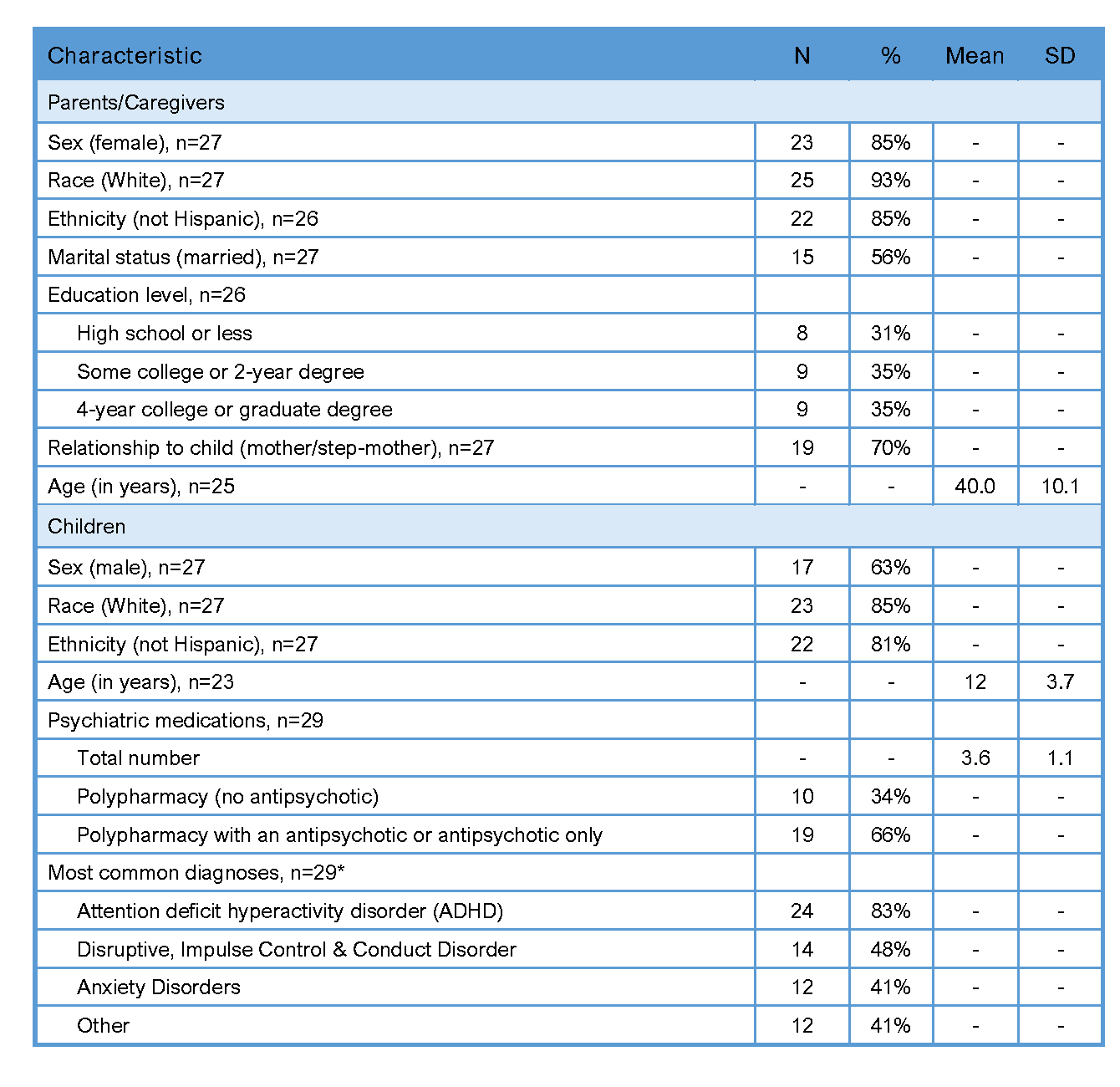 Note: SD = standard deviation, polypharmacy = 3 or more medications.
Note: SD = standard deviation, polypharmacy = 3 or more medications.*Totals exceed 100% because most participants had more than one diagnosis.

