Pediatric Therapeutics and Pharmacology
Session: Pediatric Therapeutics and Pharmacology
819 - Portable, Low-volume Microfluidic Device to Standardize Testing of Antiplatelet Agents in Neonatal and Adult Cardiac Patients
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 819.6221
Evgeni Efimenko, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, United States; Thomas Diacovo, Stony Brook Children's Hospital, Larchmont, NY, United States
- TD
Thomas Diacovo, MD (he/him/his)
Stony Brook Children's Hospital
Larchmont, New York, United States
Presenting Author(s)
Background: Thromboembolic events remain a major cause of mortality in neonates with complex CHD. Despite the plethora of approved antiplatelet agents for adults, neonatal use is limited due to insufficient PK/PD data. This is due in part to: (i) the quantity of blood that can be safely drawn, and (ii) a need for a surrogate biomarker(s) to measure drug response. Previously, we demonstrated the ability of a microfluidic (MF) system to serve as a PD biomarker for the P2Y12 antagonist cangrelor in post-op neonates with CHD [Vargas et al. JTH 2021]. However, this required an advanced microscopy system, considerable technical expertise and significant set up time, thus limiting its use in a future multicenter trial.
Objective: In preparation for a neonatal trial, we assessed the ability of a low volume MF device to detect the inhibitory effect of aspirin and the P2Y12 inhibitor clopidogrel on clot formation by enrolling adults undergoing coronary artery stenting. Results were compared to a humanized mouse model of thrombosis using platelets from the same individuals.
Design/Methods: A user friendly, portable, low volume, MF assay device was developed (FloBio LLC) (Fig 1). A vascular injury model involving mice that express the human A1 domain of von Willebrand factor to support human platelet-mediated clot formation were used for comparison [Chen et al. Blood 2014].
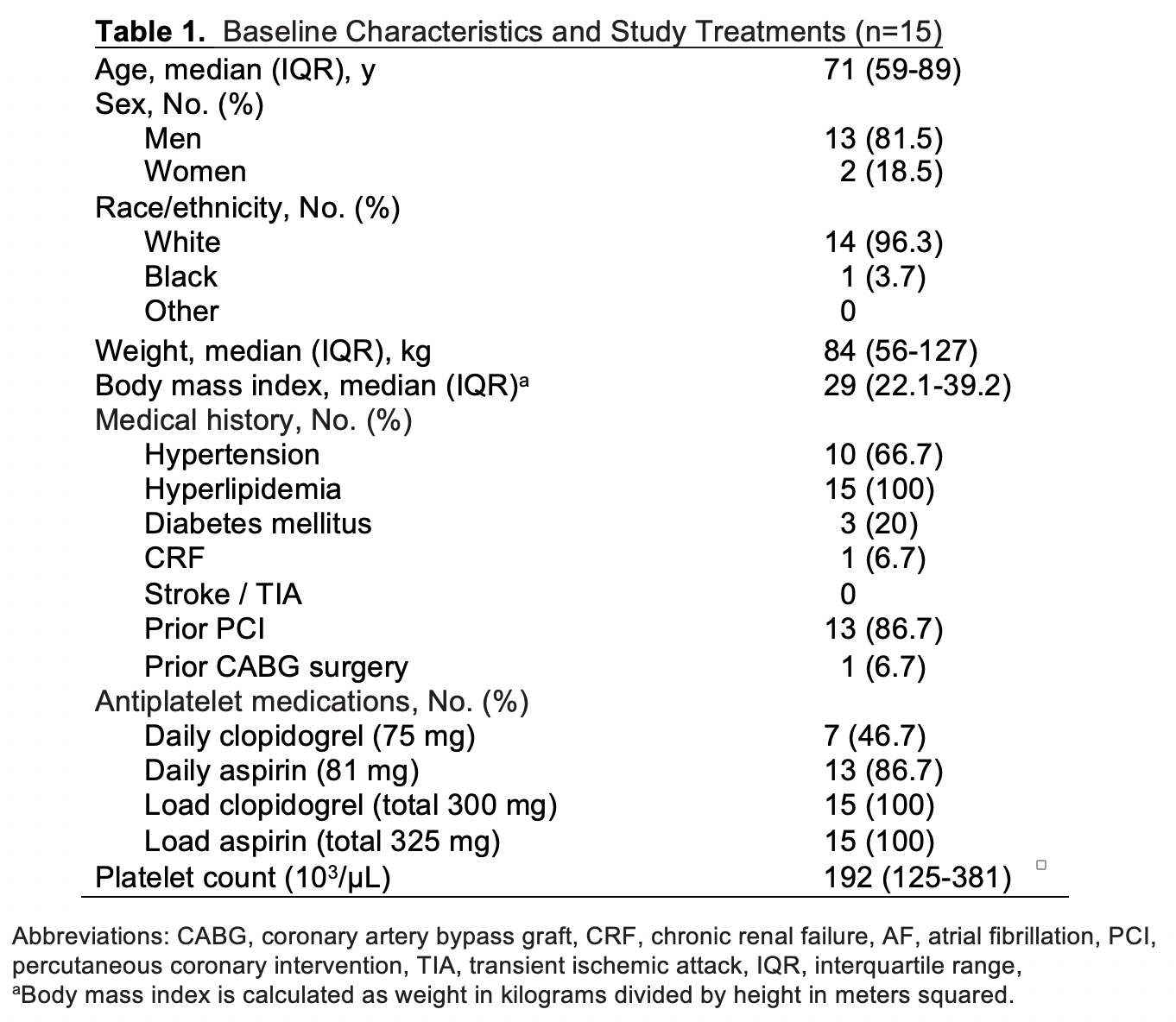
Results: Fifteen healthy donors and 15 patients (pre-loaded with 325 mg of aspirin and 300 mg of clopidogrel) were enrolled after informed consent for this IRB approved study (Table 1). With < 1 ml of blood, 8 separate platelet-mediated clotting events were imaged and analyzed by the MF device for each participant. A significant reduction in clot formation was observed for patient blood as compared to healthy donors with a normalized mean (± SD) fluorescence intensity of 17.1 (5.4; 95% CI, 14.2% to 20.1%) and 78.0 (14; (95% CI, 71% to 86.5%), respectively (Fig 2A). Consistent with MF results, patient platelets formed smaller thrombi in injured arterioles as compared to healthy donors with a mean (± SD) area of 2,241 µm2 (616; 95% CI, 2,010% to 2,471%) versus 7,147 µm2 (774.1; 95% CI, 6,858% to 7,402%), respectively (Fig 2B). Importantly, the reduction in clot formation was similar to the MF device (69% vs 78%, respectively).
Conclusion(s): We present evidence that an easy to use MF device with minimal sample volume requirement and fast processing yields results consistent with in vivo anti-platelet agent potency. Current focus is on establishing its utility as a viable PD biomarker in an upcoming clinical trial involving neonates with complex CHD
Depiction of Microfluidic Device
.png) The FloBio microfluidic device includes: (i) 8 sample well, injection molded, microfluidic chip coated with surface-immobilized collagen (stained blue for visualization), and (ii) a self-analyzer including a vacuum pump, LEDs, optics and automated control functionality to measure accumulation of Alexa Fluor-488 labeled platelets under flow conditions.
The FloBio microfluidic device includes: (i) 8 sample well, injection molded, microfluidic chip coated with surface-immobilized collagen (stained blue for visualization), and (ii) a self-analyzer including a vacuum pump, LEDs, optics and automated control functionality to measure accumulation of Alexa Fluor-488 labeled platelets under flow conditions.Baseline Characteristics of Patients and Medications
Microfluidic Phenotyping and In Vivo Assessment of PCI Patient Platelet Response to ASA and Clopidogrel
.png) Box-and-whisker plots for (A) Alexa-488 fluorophore labeled platelets accumulated on collagen coated chips using whole blood from either PCI patients administered aspirin and clopidogrel versus healthy donors (n = 8 clotting events per individual, t=900s), and (B) maximal area (µm2) occupied by calcein-labeled platelets from the same individuals after laser-induced arterial injury in the cremaster muscle of genetically modified mice possessing the A1 domain of human VWF in lieu of its murine counterpart (n=15 mice, 2 arteriole injuries per mouse per individual tested). The central box represents the values between the 10th and 90th percentiles, and the middle line is the median. Statistical significance was determined using Wilcoxon rank-sum test.
Box-and-whisker plots for (A) Alexa-488 fluorophore labeled platelets accumulated on collagen coated chips using whole blood from either PCI patients administered aspirin and clopidogrel versus healthy donors (n = 8 clotting events per individual, t=900s), and (B) maximal area (µm2) occupied by calcein-labeled platelets from the same individuals after laser-induced arterial injury in the cremaster muscle of genetically modified mice possessing the A1 domain of human VWF in lieu of its murine counterpart (n=15 mice, 2 arteriole injuries per mouse per individual tested). The central box represents the values between the 10th and 90th percentiles, and the middle line is the median. Statistical significance was determined using Wilcoxon rank-sum test.
