Neo-Perinatal Health Care Delivery: Practices and Procedures 2
Session: Neo-Perinatal Health Care Delivery: Practices and Procedures 2
476 - Probiotic receipt and neurodevelopmental outcomes in a national cohort of infants <29 weeks’ gestation
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 476.6204
Prakesh S. Shah, Mount Sinai Hospital, Toronto, ON, Canada; Nicole Bando, Unity Health Toronto, Toronto, ON, Canada; Seungwoo Lee, Mount Sinai Hospital, Toronto, ON, Canada; Kamini Raghuram, Mount Sinai Hospital, Toronto, ON, Canada; Marc Beltempo, McGill University Faculty of Medicine and Health Sciences, Monreal, PQ, Canada; Carlos Fajardo, University Of Calgary, Calgary, AB, Canada; Valerie Bertelle, Université de Sherbrooke, Sherbrooke, PQ, Canada; Andrzej Kajetanowicz, Dalhousie University Faculty of Medicine, Sydney, NS, Canada; Linh G. Ly, University of Toronto Temerty Faculty of Medicine, Toronto, ON, Canada; Rudaina Banihani, University of Toronto Temerty Faculty of Medicine, Toronto, ON, Canada; Belal Alshaikh, University of Calgary, Calgary, AB, Canada
.jpg)
Prakesh S. Shah, MD FRCPC (he/him/his)
Professor
Mount Sinai Hospital
Toronto, Ontario, Canada
Presenting Author(s)
Background: Data on neurodevelopmental and growth outcomes after exposure to probiotics are scant.
Objective: To examine the neurodevelopmental and growth outcomes following probiotic receipt in NICU among infants < 29 weeks’ gestation at 18-30 months corrected age. We hypothesized that the probiotics group will be equivalent to the no probiotics group for significant neurodevelopmental impairment (sNDI) among survivors.
Design/Methods: In this retrospective cohort study using data from the Canadian Neonatal Network (CNN) and Canadian Neonatal Follow-Up Network (CNFUN), we compared growth and neurodevelopmental outcomes of survivors who were followed up between 18-30 months corrected age born between 2014 and 2020. Probiotic administration protocols varied by unit but guidelines typically indicated provision within 24 hours of enteral feed start. Twenty of the 21 sites used multi-strain probiotic containing Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus rhamnosus; and one site used single-strain probiotic containing Lactobacillus reuteri. The primary outcome of sNDI was defined as any one of: Bayley-III cognitive, language or motor score < 70; cerebral palsy with Gross Motor Function Classification System III-V; hearing aid or cochlear implant; or bilateral visual impairment. Assuming a baseline sNDI event rate of 16% in the no probiotics group if there is truly no difference between probiotics and no probiotics groups, then 4368 patients are required to be 90% sure (beta=0.1) that the limits of a two-sided 95% confidence interval (alpha=0.025) will exclude a difference between probiotics and no probiotics of >4% (equivalence margin). The choice of 4% as the equivalence margin is based on a relative risk of 25% from a baseline risk of 16%.
Results: A total of 4533 infants (2258 in probiotics group and 2275 in no probiotics group) were evaluated (Fig 1). Baseline characteristics between groups were similar (Table 1). Results of adjusted estimates (logistic regression and propensity-score matched comparison) are in Fig 2. In the entire cohort, probiotics were equivalent to no probiotics for sNDI. In a propensity-score matched analysis of 2624 infants, probiotics showed a potential for superiority for sNDI, language score < 70 and < 85, motor score < 70 and < 85 and hearing impairment.
Conclusion(s): Probiotics were equivalent and potentially superior to no probiotics for the outcome of sNDI among surviving preterm neonates of < 29 weeks’ gestation.
Figure 1. Probiotic receipt and early childhood outcomes in a national cohort of infants <29 weeks’ gestation
.png) Abbreviations: CNFUN, Canadian Neonatal Follow-Up Network
Abbreviations: CNFUN, Canadian Neonatal Follow-Up NetworkTable 1. Demographic characteristics of followed-up infants who were and were not administered probiotics
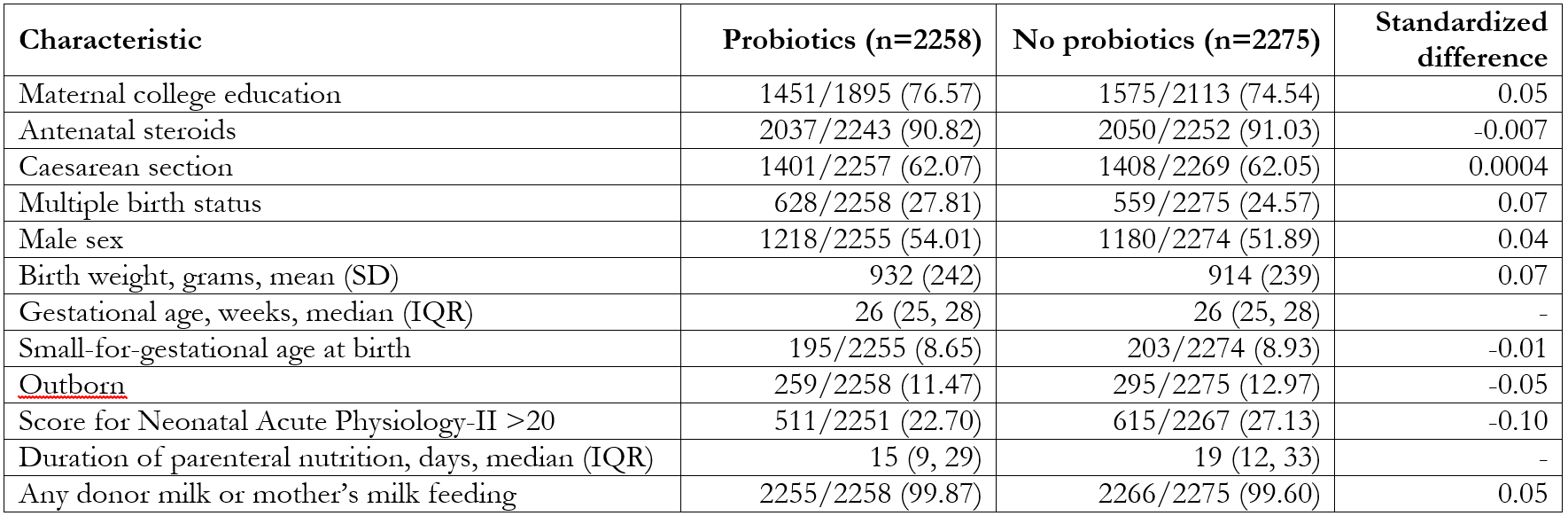 Data presented as No./Total No. (%) unless otherwise specified. Values outside of |0.1| are considered statistically significant.
Data presented as No./Total No. (%) unless otherwise specified. Values outside of |0.1| are considered statistically significant.Figure 2. Panel a. Multivariable logistic regression model (entire cohort)1 and Panel b. Multivariable regression model (Propensity score matched cohort)2
.png) Vertical dotted line in both panels represents equivalence margin of 4%.
Vertical dotted line in both panels represents equivalence margin of 4%.1Model 1: Multivariable logistic (or linear as appropriate) regression models adjusted for antenatal steroids, birth gestational age, birth weight, sex, SNAP-II score >20, delivery mode, multiple birth status, year of admission, CNN site three-level feed initiation variable, and maternal education.
2Model 2: PS matched population + adjusted for maternal education and CNN site three-level feed initiation variable. (GEE approach was used for both categorical and continuous outcomes). PS was calculated using antenatal steroids, birth gestational age, birth weight, sex, SNAP-II >20, delivery mode, multiple birth status, and year of admission as independent variables, with probiotic administration as outcome.
Abbreviations: CI, confidence interval; CNN, Canadian Neonatal Network; NDI, neurodevelopmental impairment; PS, propensity score; SNAP-II, Score for Neonatal Acute Physiology-II; sNDI, significant neurodevelopmental impairment.

