Quality Improvement/Patient Safety 1
Session: Quality Improvement/Patient Safety 1
015 - The High-Flow Holiday: A QI Initiative Using A High-Flow Nasal Cannula (HFNC) Weaning Guide To Optimize Safe HFNC Weaning in Children With Bronchiolitis
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 15.6657
Jordan E. Kurzum, St. Christopher's Hospital for Children, Philadelphia, PA, United States; James Alsop, UH Rainbow Babies & Children's Hospital, Cleveland, OH, United States; Madalein Cunningham, St. Christopher's Hospital for Children, Conshohocken, PA, United States; Elisa Vengalil, Philadelphia Department of Public Health, Philadelphia, PA, United States; Gina M. Pelullo, St. Christopher’s Hospital for Children, Warwick, PA, United States; Clara Hofman, St. Christopher's Hospital for Children, Philadelphia, PA, United States; Mathieu Wanamaker, St. Christopher's Hospital for Children, oaks, PA, United States; David Cooperberg, Drexel University College of Medicine, Philadelphia, PA, United States

Jordan E. Kurzum, MD (he/him/his)
Resident
St. Christopher's Hospital for Children
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: Bronchiolitis is traditionally treated with supportive care such as hydration, suctioning, and oxygen as needed. High-Flow Nasal Cannula (HFNC) has emerged as a core intervention. Studies have demonstrated that HFNC use outside of the Intensive Care Unit may be associated with increased length of stay (LOS). In our medium-sized free standing children’s hospital in North Philadelphia, most children with bronchiolitis requiring HFNC are admitted to the medical-surgical unit (med-surg). Prior to our study, a methodical step-wise approach was used to wean HFNC.
Objective: To reduce average time on HFNC by 20% in 12 months in patients with bronchiolitis admitted to med-surg.
Design/Methods: A Key Driver Diagram was used as a framework for this observational time series QI study (figure 1). An interprofessional team including pediatric residents, nurses, and hospitalist attendings created a HFNC weaning pathway through multiple planned sequential interventions (figure 2). We selected patients using the following inclusion criteria: age less than two years, diagnosis of bronchiolitis, required high flow nasal cannula, and admitted to med-surg. Data was extracted from the electronic health record and cohorted in monthly samples from September 2022 to October 2024.
Our primary outcome was HFNC hours. Our balancing measure was rapid response call after wean. Data were analyzed using Excel and QIMacros; control charts and established rules for detecting special cause variation were applied.
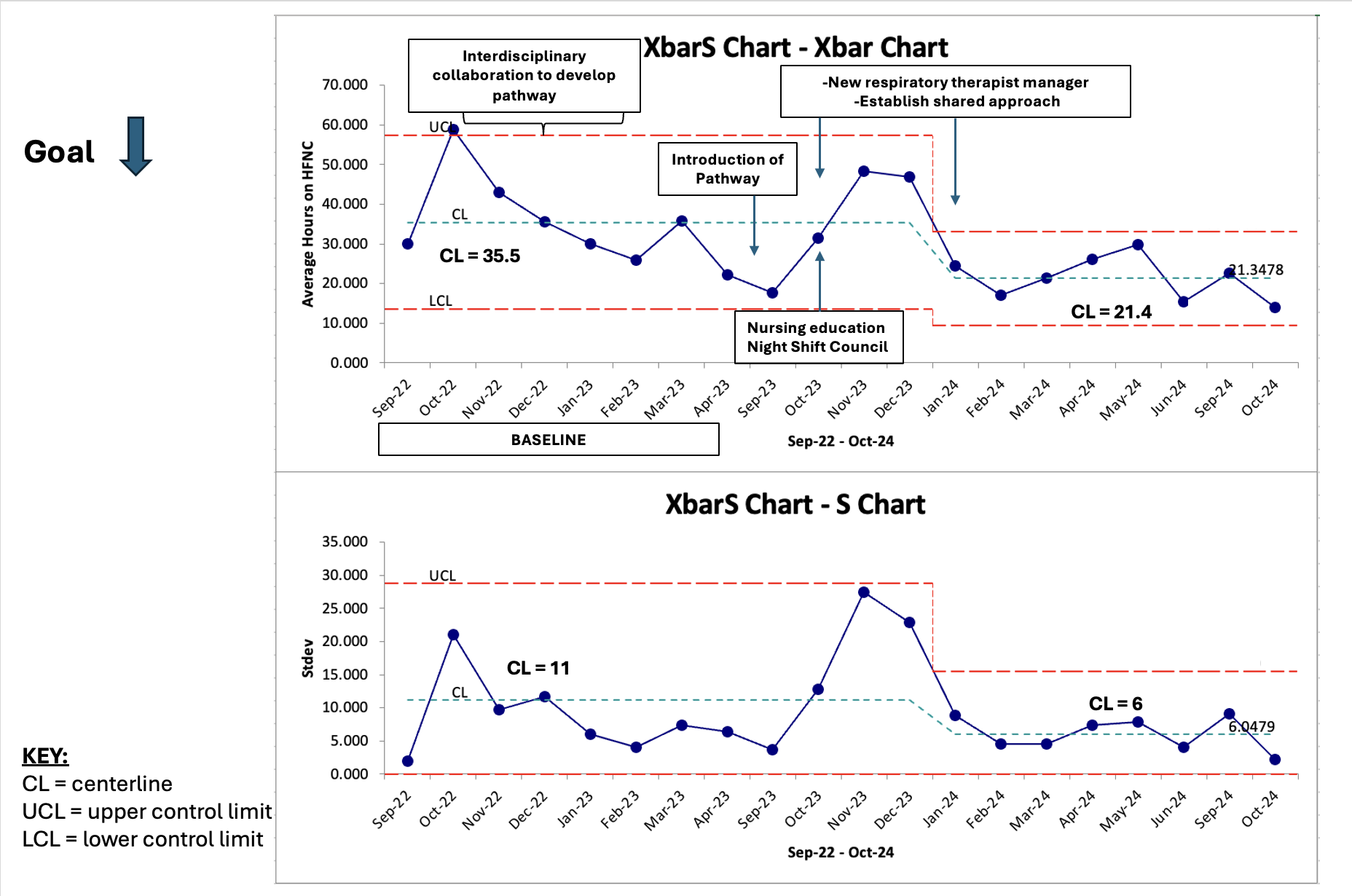
Results: 714 patients met inclusion criteria. An Xbar-S statistical process control chart was plotted based on mean HFNC hours (figure 3). During the study period the average duration of HFNC decreased by 39.8% (35.5 hours to 21.4 hours). Special cause variation was demonstrated with eight consecutive points below the mean. No rapid responses were called on patients weaned via pathway use. LOS remained unchanged. There were no statistically significant differences in mean hours on HFNC (p = 0.89) or mean LOS (0.93) controlling for race and ethnicity and mean hours on HFNC (p = 0.20) when controlling for language. Mean LOS when controlling for language was statistically significant (p = 0.0013).
Conclusion(s): We achieved our aim. Keys to success included development of a pathway including the “High-Flow Holiday”, interprofessional teamwork, and near real-time review of barriers to implementation. Next steps include use of EHR-integrating pathway tracking, further examination of disparities in LOS based on preferred language, and rational subgroup analysis for high risk groups (premature infants and infants < 2 months of age).
Figure 1. Key Driver Diagram
.png)
Figure 2. HFNC Weaning Pathway
.png)
Figure 3. X-bar and S Statistical Process Control Chart