Allergy, Immunology, and Rheumatology 2
Session: Allergy, Immunology, and Rheumatology 2
655 - The Current State of Systemic Juvenile Idiopathic Arthritis Management
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 655.6646
Jasmine Oh, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Mariana Correia Marques, National Institute Of Arthritis & Musculoskeletal & Skin Diseases, Bethesda, MD, United States; Susan Shenoi, Seattle Children's, Seattle, WA, United States; Karen S. Onel, HSS, New York, NY, United States; Rebecca Trachtman, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- JO
Jasmine Oh, BA
Clinical Research Coordinator
Icahn School of Medicine at Mount Sinai
New York, New York, United States
Presenting Author(s)
Background: Systemic Juvenile Idiopathic Arthritis (sJIA) is a subset of Juvenile Idiopathic Arthritis (JIA), and accounts for 10 to 15% of children with JIA in North America [1,2]. Currently, there is no cure for sJIA and the goal of treatment is to reduce symptoms and treat the underlying inflammation [2]. Current practice around evaluation, screening for associated complications, and appropriate treatments for sJIA is variable and is not standardized. Further, recognition of life-threatening forms of sJIA-Associated Lung Disease (sJIA-LD) is increasing and understanding of this phenotype is rapidly evolving, with increased reports over the past decade [3].
Objective: Because the landscape of treatment for sJIA is changing, we aimed to identify current practices around sJIA evaluation and management, focusing on screening and treatment of sJIA-LD amongst pediatric rheumatologists (PR), to facilitate future research in this area.
Design/Methods: PR who treat sJIA were recruited to complete a survey through an announcement included in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) newsletter. The newsletter was sent to CARRA members through the listserv. We collected: demographic data, information on clinician’s screening and treatment plans for patients with sJIA, including sJIA-LD.
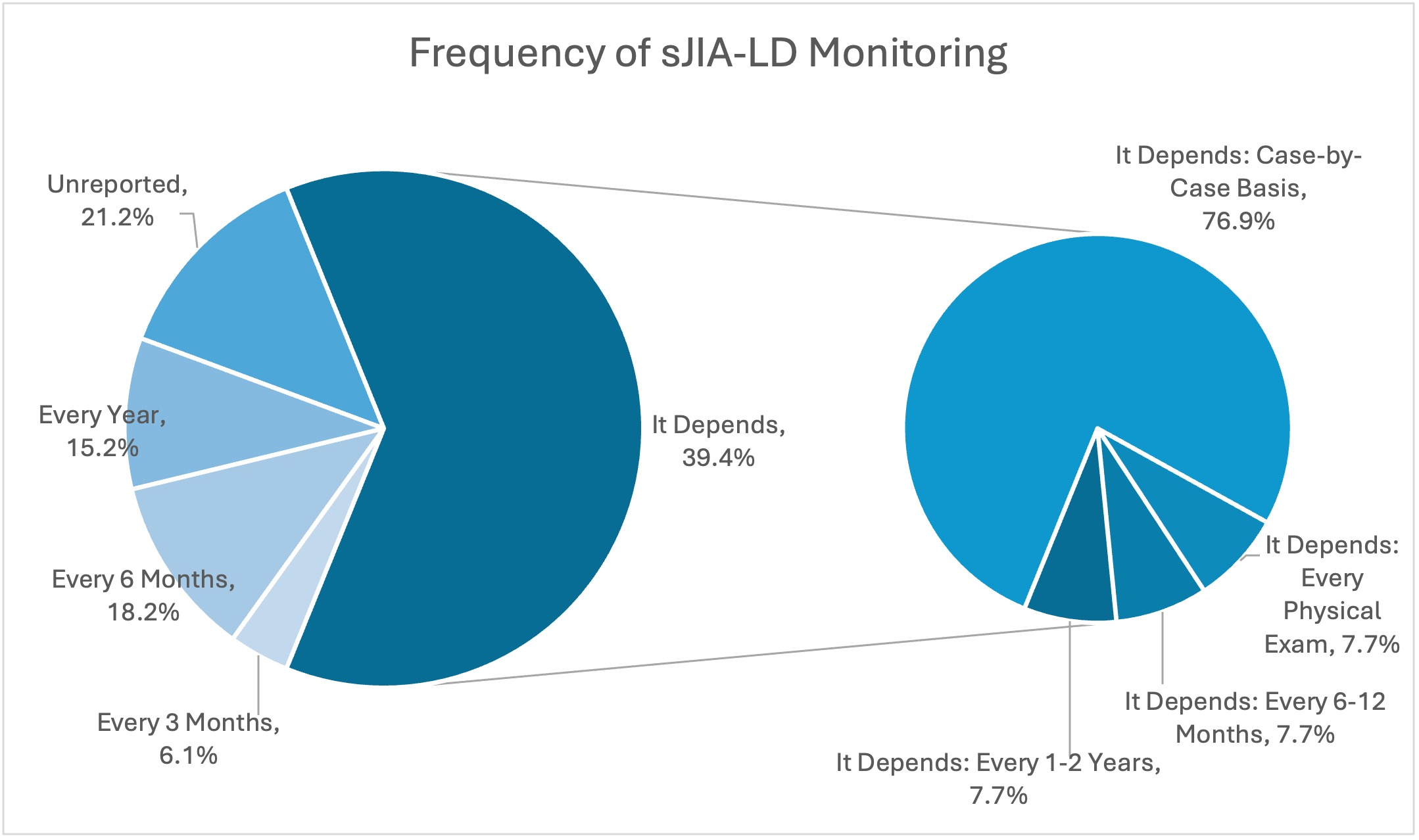
Results: 33 PR responded. Only 30.4% of PR perform HLA-DRB1 screening for sJIA patients, at varying points in disease course. The most common medication used to treat sJIA without LD, was IL-1 blockade (88%), though PR continued to utilize other biologic and non-biologic agents as well. 40% of PR reported that they regularly screen patients for sJIA-LD, whereas 40% of providers reported that it is done on a case-by-case basis. When sJIA-LD diagnosis was confirmed, 76% of providers initially prescribed non-biologic disease-modifying antirheumatic drugs, though corticosteroids and biologic agents were employed as well. As shown in Graph 1, providers varied substantially in their monitoring timeline for patients with sJIA-LD, with specific answers ranging 3 months to one year, though 30% stated that this would be determined on a case-by-case basis.
Conclusion(s): Current practices around screening, monitoring and treatment of children with sJIA-LD are not uniform and often depend on the provider’s assessment of the individual patient case. Based on our findings, it is clear that further research is needed to identify best practice for evaluation and treatment of children with sJIA, especially sJIA-LD.
Graph 1. Frequency of sJIA-LD Monitoring