Neonatal Pulmonology - Basic/Translational Science 2
Session: Neonatal Pulmonology - Basic/Translational Science 2
233 - Suppression of microRNA-34a attenuates hyperoxia-induced senescence in mouse lung
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 233.6606
Hajime Maeda, School of Medicine, Fukushima Medical University, Fukushima, Fukushima, Japan; Hayato Go, Fukushima Medical Iniversity, Fukushima, Fukushima, Japan; Nozomi Kashiwabara, Fukushima Medical University,, Fukushima, Fukushima, Japan; Hongwei Yao, Providence VA Medical Center, Providence, RI, United States; Phyllis A.. Dennery, The Warren Alpert Medical School of Brown university, Providence, RI, United States
- HM
Hajime Maeda, MD, PhD
Assistant professor
School of Medicine, Fukushima Medical University
Fukushima, Fukushima, Japan
Presenting Author(s)
Background: Bronchopulmonary dysplasia (BPD) is a chronic lung disease in premature infants, characterized by alveolar dysplasia and impaired vascularization. Supplemental oxygen and mechanical ventilation, commonly used in premature infants, may result in BPD. Previous studies have shown that miR-34a is increased in the lung of patients with BPD and hyperoxia-exposed mice. We have shown that exposure to high concentration of oxygen (95% O2/5%CO2; hyperoxia) increased miR-34a-5p leading to senescence in cultured lung epithelial cells. However, it is unclear whether increased miR-34 expression contributes to hyperoxia-induced senescence in the lung.
Objective: To investigate whether hyperoxia increases miR-34a levels, leading to cellular senescence in mouse lung.
Design/Methods: Newborn mice ( < 12 h old) were exposed to hyperoxia (>95% O2) for 3 days and allowed to recover in room air until postnatal day (PND) 7. We intranasally administered 4 µl of miR-34a inhibitor (20 µM concentration) or PBS as control at PND0 and PND2 during hyperoxia exposure. At PND3, we evaluated miR-34a expression and lung senescence biomarkers, including p53, p21, p16, PAI-1, Cxcl2, and IL-1β gene expression.
Results: Hyperoxic exposure significantly increased lung miR-34a expression (3.6-fold). Administration of the miR-34a inhibitor suppressed its expression. Hyperoxia as associated with increased markers of senescence p21, p16, and PAI-1 mRNA at PND3 (8.6-fold, 1.6-fold, and 2.5-fold, respectively). Lung p21 protein levels were also increased at PND3 in mice exposed to hyperoxia as neonates. These senescence biomarkers were significantly reduced after administration of miR-34a inhibitor.
Conclusion(s): Hyperoxia increases miR-34a, leading to senescence in mouse lung. Therefore, inhibiting hyperoxia-induced senescence via the suppression of miR-34a may provide a novel therapeutic strategy to mitigate the detrimental consequences of hyperoxia in the neonatal lung.
miR-34a-5p inhibitor reduces hyperoxia-induced senescence in the lung of mice.
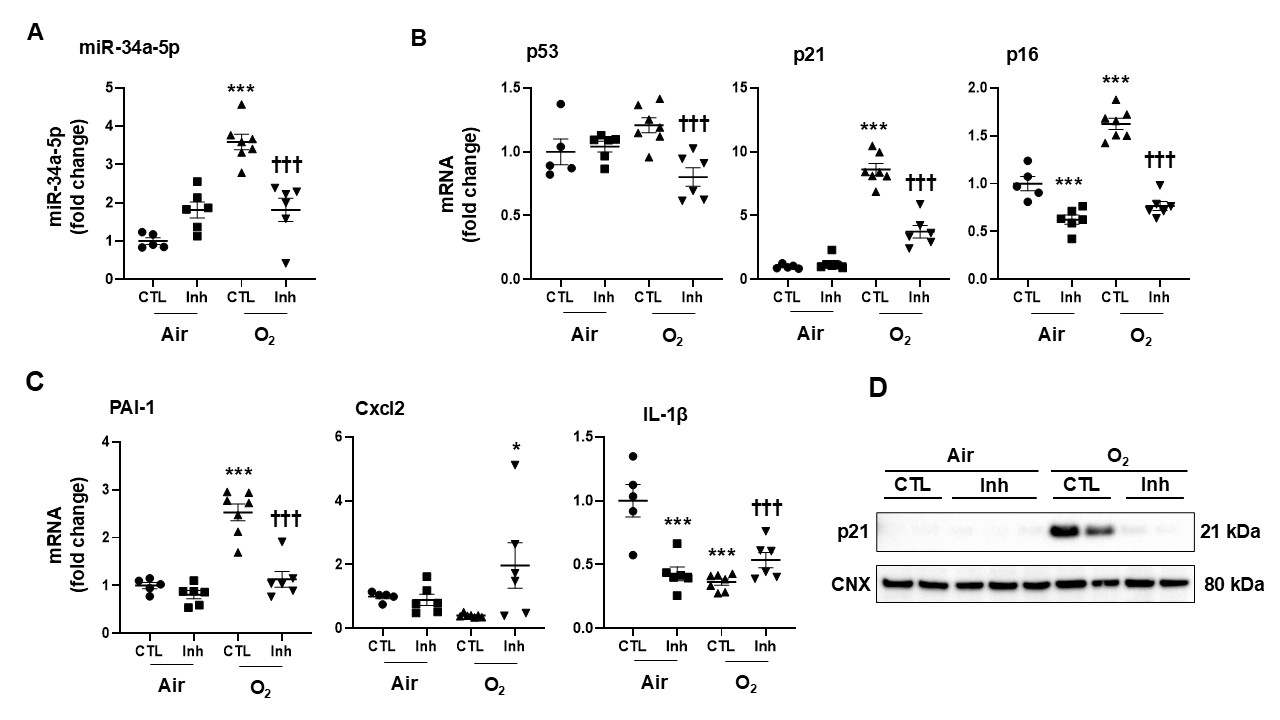
miR-34a-5p inhibitor reduces hyperoxia-induced senescence in the lung of mice.


