Back
Background: Noonan syndrome (NS) is a genetic disorder characterized by distinct facial features, cardiovascular defects, developmental delay, and increased risk for cancer. NS is inherited in an autosomal dominant fashion and has been associated with dysregulation of the RAS/MAPK signaling pathway. SOS1 is the second most common gene associated with NS. We present a detailed series of patients with SOS1-related NS, as well as a literature review of oncologic manifestations in the setting of SOS1-related NS.
Objective: To describe the myriad phenotypic presentations and increased cancer incidence of SOS1 NS, highlighting the role of individualized medicine and continued follow-up care of these patients.
Design/Methods: We retrospectively identified all patients diagnosed with NS with pathogenic or likely pathogenic SOS1 variants at Mayo Clinic between 2000-2023.
Results: We identified 9 patients with SOS1-related NS (Table 1). Congenital heart defects including aortic dilation, bicuspid aortic valve, atrial septal defect, mitral valve abnormalities, and partial anomalous pulmonary venous return were present in 3 (33%) patients. Arrhythmias were reported in 2 (22%) patients, with 1 aborted sudden cardiac arrest related to ventricular fibrillation. Hypertrophic cardiomyopathy was noted only in 1 (11%) patient. Extracardiac findings favored facial and ectodermal phenotypes with lower rates of intellectual disability and musculoskeletal findings. Oncologic manifestations were present in 2 (22%) patients with distinct variants. Giant cell lesion (GCL) of jaw and pigmented villonodular synovitis (PVNS) was reported (c.797C>A) while GCL of skull along with meningioma was described in another patient [c.2510+42510+7(del)]; this is the first reported incidence of cancer associated with these specific NS variants. A detailed literature review revealed 29 patients with SOS1-related NS who had at least one cancer (Table 2) with proclivity for GCL (45%) followed by compound odontoma (21%), embryonal rhabdomyosarcoma (14%), B-cell acute lymphoblastic leukemia (7%), PVNS (7%), colorectal cancer (3%), and Sertoli cell tumor (3%).
Conclusion(s): A variety of cardiac and extracardiac phenotypes were reported heterogeneously among patients with SOS1 NS, suggesting the need for continued periodic surveillance and cardiac monitoring. We hypothesize that dysregulation of the RAS/MAPK signaling pathway may contribute to GCL generation. Our findings suggest that patients with SOS1 NS may benefit from implementing screening evaluations for concurrent malignancies with special attention to GCL.
Table 1
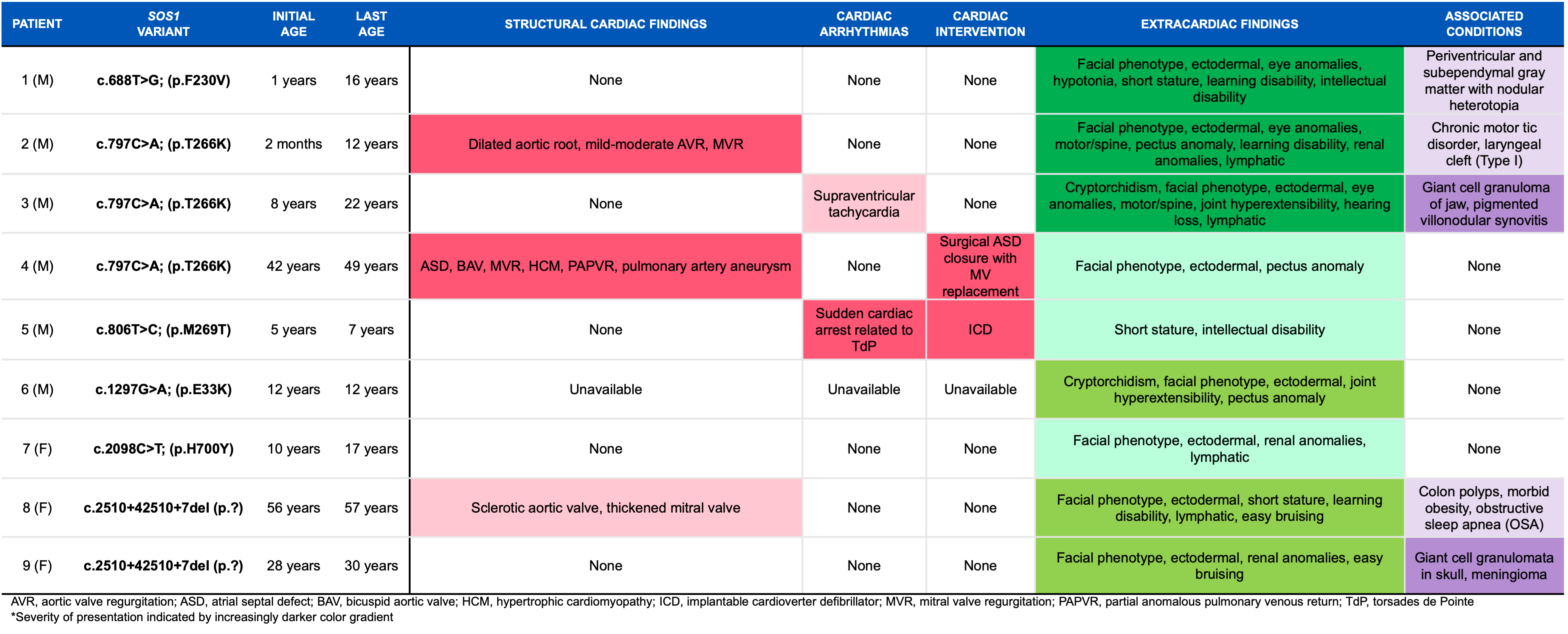 Detailed characteristics of patients with SOS1 Noonan Syndrome.
Detailed characteristics of patients with SOS1 Noonan Syndrome.
Table 2
.png) Incidence of cancers in association with SOS1 across the literature.
Incidence of cancers in association with SOS1 across the literature.
Table 1
 Detailed characteristics of patients with SOS1 Noonan Syndrome.
Detailed characteristics of patients with SOS1 Noonan Syndrome.
Table 2
.png) Incidence of cancers in association with SOS1 across the literature.
Incidence of cancers in association with SOS1 across the literature.
Genomics/Epigenomics 2
Session: Genomics/Epigenomics 2
391 - Cardiac Manifestations & Malignancies in SOS1 Related Noonan Syndrome: Case Series & Literature Review
Friday, April 25, 2025
5:30pm – 7:45pm HST
Michael A. Cirelli, Mayo Clinic Alix School of Medicine, Scottsdale, AZ, United States; Rabia Javed, Mayo Clinic, Rochester, MN, United States; Lisa A. Schimmenti, Mayo Clinic Alix School of Medicine, Rochester, MN, United States; Jonathan N. Johnson, Mayo Clinic Children's Center, Rochester, MN, United States; Heidi Connolly, Mayo Clinic, Rochester, MN, United States; Zachary Wilson, Mayo Clinic Children's Center, Rochester, MN, United States; Ahmad Al-Huniti, Mayo Clinic, Rochester, MN, United States; Susan G.. MacLellan-Tobert, Gundersen Health System, Onalaska, WI, United States; Talha Niaz, Mayo Clinic, Rochester, MN, United States; Whitney S. Thompson, Mayo Clinic, Rochester, MN, United States

Michael A. Cirelli, Jr., BA (he/him/his)
Medical Student
Mayo Clinic Alix School of Medicine
Scottsdale, Arizona, United States
Presenting Author(s)
Background: Noonan syndrome (NS) is a genetic disorder characterized by distinct facial features, cardiovascular defects, developmental delay, and increased risk for cancer. NS is inherited in an autosomal dominant fashion and has been associated with dysregulation of the RAS/MAPK signaling pathway. SOS1 is the second most common gene associated with NS. We present a detailed series of patients with SOS1-related NS, as well as a literature review of oncologic manifestations in the setting of SOS1-related NS.
Objective: To describe the myriad phenotypic presentations and increased cancer incidence of SOS1 NS, highlighting the role of individualized medicine and continued follow-up care of these patients.
Design/Methods: We retrospectively identified all patients diagnosed with NS with pathogenic or likely pathogenic SOS1 variants at Mayo Clinic between 2000-2023.
Results: We identified 9 patients with SOS1-related NS (Table 1). Congenital heart defects including aortic dilation, bicuspid aortic valve, atrial septal defect, mitral valve abnormalities, and partial anomalous pulmonary venous return were present in 3 (33%) patients. Arrhythmias were reported in 2 (22%) patients, with 1 aborted sudden cardiac arrest related to ventricular fibrillation. Hypertrophic cardiomyopathy was noted only in 1 (11%) patient. Extracardiac findings favored facial and ectodermal phenotypes with lower rates of intellectual disability and musculoskeletal findings. Oncologic manifestations were present in 2 (22%) patients with distinct variants. Giant cell lesion (GCL) of jaw and pigmented villonodular synovitis (PVNS) was reported (c.797C>A) while GCL of skull along with meningioma was described in another patient [c.2510+42510+7(del)]; this is the first reported incidence of cancer associated with these specific NS variants. A detailed literature review revealed 29 patients with SOS1-related NS who had at least one cancer (Table 2) with proclivity for GCL (45%) followed by compound odontoma (21%), embryonal rhabdomyosarcoma (14%), B-cell acute lymphoblastic leukemia (7%), PVNS (7%), colorectal cancer (3%), and Sertoli cell tumor (3%).
Conclusion(s): A variety of cardiac and extracardiac phenotypes were reported heterogeneously among patients with SOS1 NS, suggesting the need for continued periodic surveillance and cardiac monitoring. We hypothesize that dysregulation of the RAS/MAPK signaling pathway may contribute to GCL generation. Our findings suggest that patients with SOS1 NS may benefit from implementing screening evaluations for concurrent malignancies with special attention to GCL.
Table 1
 Detailed characteristics of patients with SOS1 Noonan Syndrome.
Detailed characteristics of patients with SOS1 Noonan Syndrome.Table 2
.png) Incidence of cancers in association with SOS1 across the literature.
Incidence of cancers in association with SOS1 across the literature.Table 1
 Detailed characteristics of patients with SOS1 Noonan Syndrome.
Detailed characteristics of patients with SOS1 Noonan Syndrome.Table 2
.png) Incidence of cancers in association with SOS1 across the literature.
Incidence of cancers in association with SOS1 across the literature.
