Global Neonatal & Children's Health 2
Session: Global Neonatal & Children's Health 2
738 - Evaluation of a Novel Reprocessed bCPAP System on Sepsis Rates among Preterm Neonates with Respiratory Distress: A Randomized Controlled Trial
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 738.4692
Sarah Badin, Vayu Global Health Foundation, Boston, MA, United States; Navid P. Roodaki, ITRMC, San Fernando City, La Union, Philippines; Rochelle G.. Abila-Cariaga, Ilocos Training and Regional Medical Center, Ramon, Isabela, Philippines; Thomas F. Burke, Harvard University, Medford, MA, United States; Daisy Evangeline C. Garcia, Philippine Society of Newborn Medicine, San Fernando City, La Union, Philippines

Sarah Badin, MD (she/her/hers)
Postdoctoral Research Fellow
Vayu Global Health Foundation
Boston, Massachusetts, United States
Presenting Author(s)
Background: Bubble CPAP (bCPAP) is highly effective in the treatment of respiratory distress syndrome of prematurity and other causes of newborn respiratory insufficiency. However, bCPAP devices are scarce in low-and-middle income countries. To increase global access a novel bCPAP system was developed that is in use across multiple countries. The bCPAP system does not use electricity, it is portable and easy to use, and designed to be cleaned, disinfected, and reused.
Objective: This study aimed to evaluate whether use of cleaned and disinfected bCPAP systems increases the rate of sepsis.
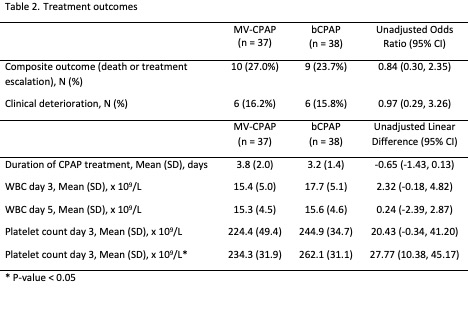
Design/Methods: A post hoc analysis of a randomized controlled trial was conducted that compared a mechanical ventilator driven CPAP device (MV-CPAP) with single-use circuits to a reusable bCPAP system (Vayu bCPAP) that was reprocessed after each use. Consecutive eligible neonates treated with CPAP within 6 hours of birth were randomized to one of the two CPAP devices. The primary outcome was a composite of treatment escalation or death. Secondary outcomes included clinical deterioration, time on CPAP, white blood cell counts, and platelet counts.
Results: Seventy-five neonates were randomized to the two CPAP treatment arms. No significant differences in death (5 vs 4), escalation of care (10 vs 9), and the composite outcome (OR = 0.84; 95% CI: 0.30 – 2.35, p = 0.743) were detected in the MV-CPAP and Vayu bCPAP groups respectively. There were no clinically significant differences in any of the secondary outcomes.
Conclusion(s): Use of a reprocessed bCPAP system designed to increase global access to CPAP did not increase rates of neonatal sepsis.
Flowchart of patients recruited in the trial
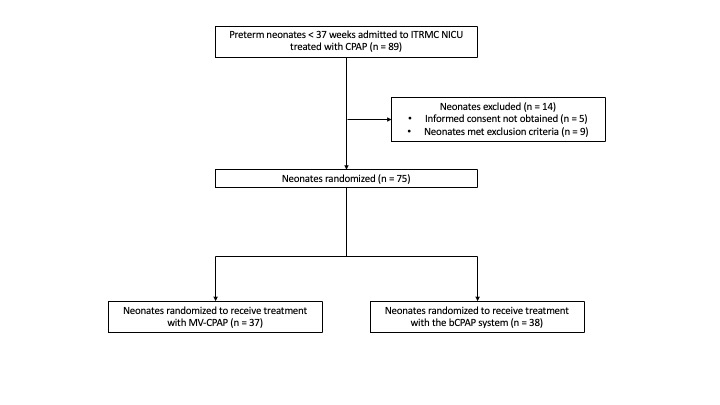
Baseline demographic and clinical characteristics
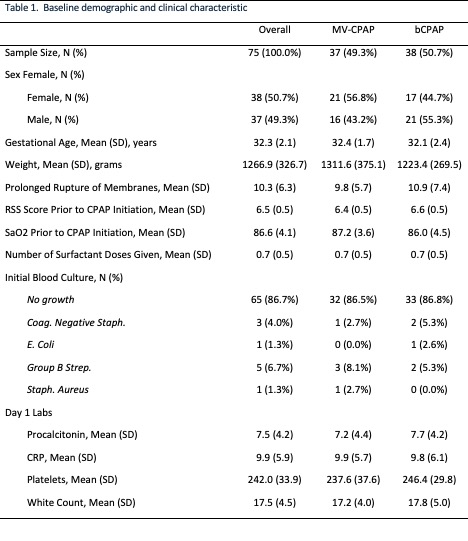
Treatment outcomes