Emergency Medicine 3
Session: Emergency Medicine 3
437 - Derivation and validation of a clinical decision rule to discriminate bacteremia from contaminants among children with a positive blood culture in the emergency department
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 437.4427
Jocelyn Gravel, Université de Montreal, Montréal, PQ, Canada; Charlotte Grandjean-Blanchet, CHU Sainte-Justine, Montreal, PQ, Canada; Alino Demean Loghin, Universite de Montreal Faculty of Medicine, Montreal, PQ, Canada; Brandon Noyon, Department of Pediatric Emergency Medicine, Centre Hospitalier Universitaire Sainte-Justine, Montréal, PQ, Canada; Olivia Ostrow, Hospital for Sick Children, Toronto, ON, Canada; Emilie Vallieres, CHU Sainte-Justine, Montreal, PQ, Canada; Soha Rached-Dastous, Universite de Montreal Faculty of Medicine, Montreal, PQ, Canada

Olivia Ostrow, MD (she/her/hers)
Staff Physician and Director of Quality and Safety, Emergency Medicine
Hospital for Sick Children
Toronto, Ontario, Canada
Presenting Author(s)
Background: Blood cultures are commonly performed to rule out bacteremia in children seen in the emergency department (ED). While a true bacteremia is potentially life-threatening requiring urgent treatment, many cases of positive blood cultures are contaminants leading to unnecessary antibiotic exposures and hospitalizations.
Objective: We aimed to derive and validate a clinical decision rule to discriminate bacteremia from contaminants among children seen in the ED with a preliminary positive blood culture.
Design/Methods: This study includes two retrospective cohorts of children with positive blood cultures from a Canadian pediatric ED from January 2018 until December 2022 (derivation cohort) and January 2023 to May 2024 (validation cohort). The primary outcome was true bacteremia defined using a two-step standardized approach. Potential predictors of bacteremia were derived from a literature review and expert consensus. We used Classification and Regression Tree (CART) models to derive a highly sensitive clinical decision rule to distinguish true bacteremia from contamination. Validity was assessed by measuring the proportion of children with true bacteremia who were classified at high or moderate risk by the clinical decision rule (sensitivity) and the proportion of contaminants that were classified at low risk by the rule (specificity).
Results: A total of 574 children, including 285 cases of bacteremia were included in the derivation phase, and 173 (including 83 bacteremia) in the validation cohort. Children at high risk of bacteremia were identified based on the initial Gram stain (Gram positive bacteria in pair or chain, or all Gram negative bacteria). In the absence of high-risk Gram stain criteria, children were at moderate risk if they had any one of three risks factors. Children without any of the four criteria were classified as low risk. This clinical decision rule demonstrated a sensitivity of 100% (95%CI: 98-100%) and specificity of 65% (95%CI: 59-70%) in the derivation cohort and a sensitivity of 99% (95%CI: 94-100%) and a specificity of 60% (95%CI: 50-70%) in the validation cohort. Applying the rule to the 43 children initially discharged in the validation cohort would decrease the number of admissions from 34 to 21 without missing a true bacteremia case.
Conclusion(s): We created a highly sensitive clinical decision rule to identify true bacteremia among children seen in the ED with a preliminary positive blood culture. The use of this clinical decision rule will decrease unnecessary testing and antibiotics in a subset of patients while ensuring treatment of children at high risk of true bacteremia
CHU Sainte-Justine Clinical Decision Rule for Bacteremia
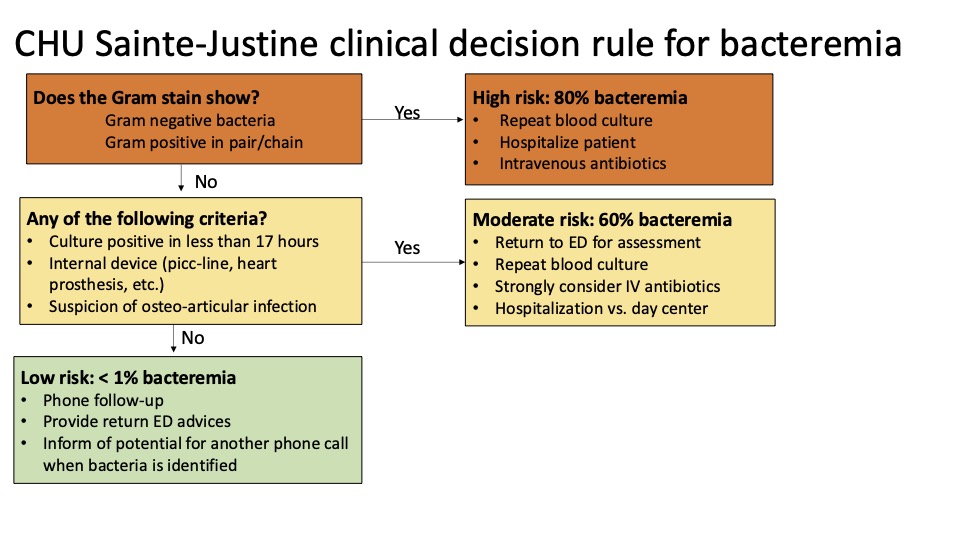
Derivation of the CHU Sainte-Justine Clinical Decision Rule for Bacteremia
Slide1.jpeg
Clinical management of patients discharged at the index visit at the time of positive blood culture
Slide1.jpeg

