Neonatal Pulmonology - Basic/Translational Science 1
Session: Neonatal Pulmonology - Basic/Translational Science 1
207 - Creation of a novel neonatal piglet model of acute lung injury to enhance therapeutic translation for premature infants at risk of bronchopulmonary dysplasia
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 207.4407
Marc-Olivier Deguise, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada; Ewa Henckel, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON, Canada; Doreen Engelberts, Ottawa Hospital Research Institute, Alcove, PQ, Canada; Shumei Zhong, ottawa hospital research institute, Ottawa, ON, Canada; Arul Vadivel, Ottawa Hospital Research Institute, Ottawa, ON, Canada; Bernard Thébaud, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada

Marc-Olivier Deguise, MD/PhD (he/him/his)
Neonatal-Perinatal Fellow
Children's Hospital of Eastern Ontario
Ottawa, Ontario, Canada
Presenting Author(s)
Background: The most premature infants (22+ weeks gestation) may now survive. One third will develop bronchopulmonary dysplasia (BPD). BPD has important long-term consequences and currently no cure is available. We pioneered umbilical cord derived mesenchymal stromal cells (UCMSC) as a therapy for BPD. Preliminary safety assessment in preterms seems promising in our recent phase I trial. Optimizing delivery is needed to ensure successful translation of UCMSC. Many clinical trials fail due to poor preclinical studies. MSC beneficial effect in BPD models has been mostly restricted to rodents.
Objective: To develop a neonatal piglet model of acute lung injury (ALI) recreating the events faced by the preterm lung to better understand early pathogenic effectors of BPD, identify and test therapeutics for clinical translation.
Design/Methods: We built a one-day (6-hour) ALI model in newborn piglets that relies on “multi-hit” consisting of surfactant lavage/removal (akin of respiratory distress syndrome), hyperoxia (free radicals), mechanical ventilation and administration of lipopolysaccharide (enhanced inflammation). We used various biochemical (cytokine levels, neutrophil activation (myeloperoxidase - MPO)), histological (hematoxylin and eosin (H&E)), and physiological (vitals and lung function such as PaO2/FiO2 - P/F ratio, oxygenation index – OI, compliance, alveolar-arterial (A-a) gradient ) measures to characterize the lung injury as per the American Thoracic Society criteria for ALI.
Results: We describe a successful fulfillment of the American Thoracic Society criteria for ALI (minimum 3 of 4). Alongside cardiovascular stability, our multi-hit model generates a severe ALI, with some recovery into moderate ALI as per P/F ratio (100-200 mmHg), OI (8-12), accompanied by functional deficits in respiratory system compliance (Crs) by 50%. Blinded evaluation of H&E-stained lung identified heterogenous histologic damage with proteinaceous debris filling the airspaces and alveolar septal thickening. The inflammatory process is marked by neutrophil influx in alveolar and interstitial space on H&E (confirmed in bronchoalveolar lavage fluid), increased MPO and elevated inflammatory cytokines. To ensure reversibility of the injury, clinically common therapeutic strategies (such as surfactant and dexamethasone - dex) were used as positive control. Dex and surfactant partially reversed in some but not all parameters identified in our multi-hit model.
Conclusion(s): This multi-hit model will be critical in deciphering early BPD triggers, facilitate therapeutic evaluation and delivery optimization for effective clinical translation.
Experimentation schematic of the neonatal piglet acute lung injury model
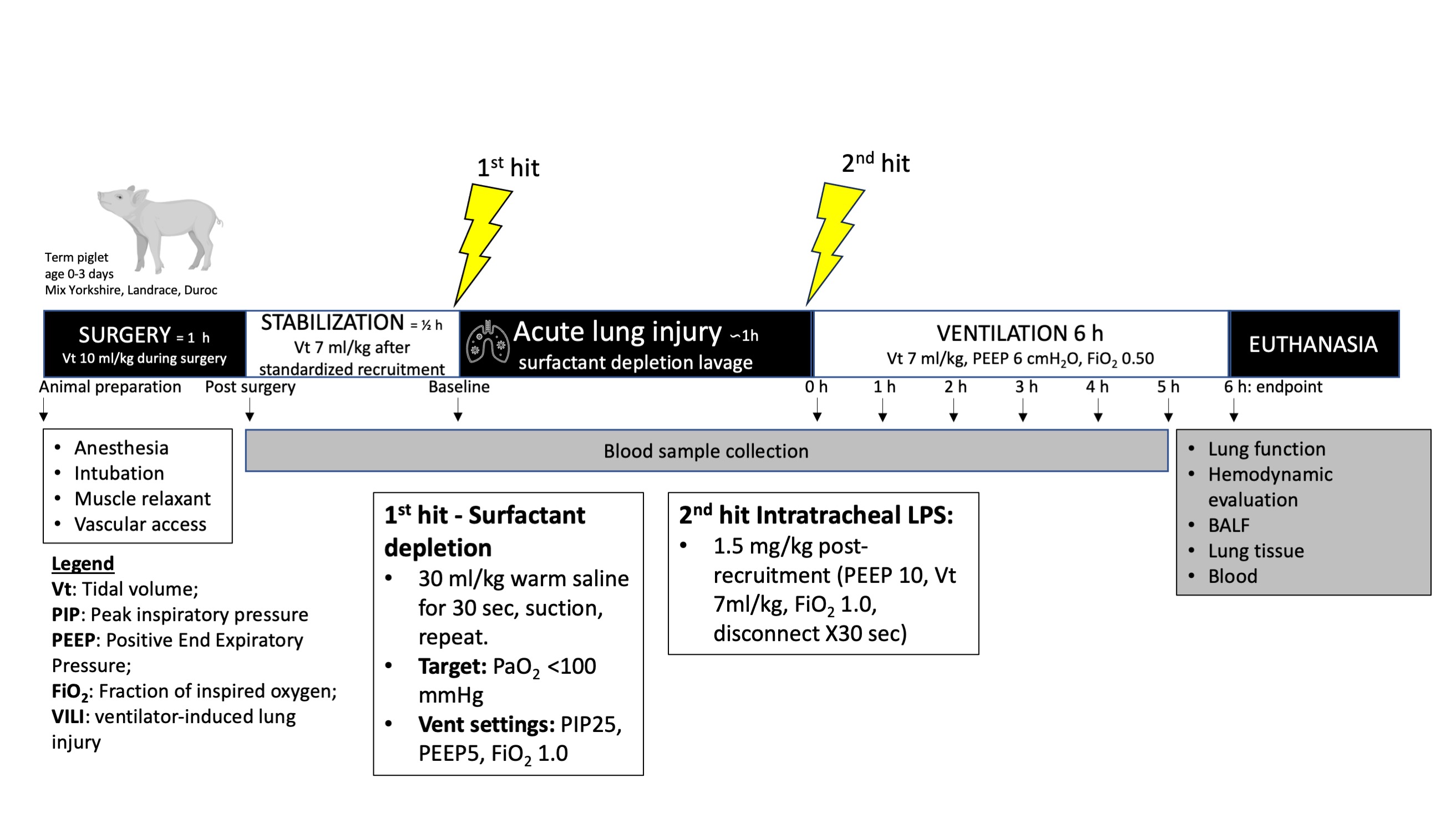 Timeline including the major steps involved in the creation of our new multi-hit acute lung injury model in neonatal piglets.
Timeline including the major steps involved in the creation of our new multi-hit acute lung injury model in neonatal piglets.
