Genomics/Epigenomics 2
Session: Genomics/Epigenomics 2
394 - Differences in circulating inflammatory cell makeup and gene expression at single-cell resolution in children with and without obesity
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 394.4276
Kathryn E. Kyler, Children's Mercy Kansas City, Kansas City, MO, United States; Bridgette L. Jones, Childrens' Mercy, Kansas City, MO, United States; Elin Grundberg, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States

Kathryn E. Kyler, MD, MSc
Assistant Professor of Pediatrics, Hospital Medicine
Children's Mercy Kansas City
Kansas City, Missouri, United States
Presenting Author(s)
Background: Differences in baseline inflammation exist in children with obesity which may impact disease risk, phenotype, development of comorbidities and drug response. However, cellular-level transcriptomic differences in inflammation between children with and without obesity are not well understood and could aid in understanding the pathophysiology underlying obesity-related inflammation and its sequelae.
Objective: We aimed to determine differences in peripheral blood mononuclear cell (PBMC) makeup and cellular-level genetic expression between children with and without obesity. We also examined differences across other biological factors (i.e., age/sex).
Design/Methods: Utilizing PBMC samples from children with obesity (n=12) undergoing bariatric surgery and age/sex matched controls (n=12), we applied single-cell RNA-sequencing (10X Genomics) to measure cellular composition and transcriptional profile of PBMCs. We used Wilcoxon rank sum to test differences in PBMC cell type proportions between groups with and without obesity and across age and sex. Differential expression in inflammatory genes between individuals with and without obesity were compared using pseudobulk analysis and Wilcoxon rank sum testing with p-values adjusted by Bonferroni correction.
Results: PBMC samples from children with obesity had differing PBMC cellular makeup, with larger proportions CD4+ T central memory (Tcm) cells (mean 17%, SD=5.7) compared to controls (12%, SD=4.5; p=0.03; Fig. 1). There was a positive correlation between body mass index (BMI) and CD4+Tcm cell proportion (r2=0.25; Fig. 2). No differences existed in CD4+ Tcm cell proportion between males (n=8) and females (n=16)(p=0.07). We found no correlation between CD4+Tcm cell proportion and age (range 11-18 years; r2=0.03). CD4+ Tcm cells were characterized by high interleukin 7 receptor (IL7R) expression compared to all other cell types (average log2fold difference 1.36, adjusted p-value < 6e-300). IL7R expression within the CD4+ Tcm cell cluster was also higher in the group with obesity compared to those without obesity (average log2fold difference 0.53, adjusted p-value=8.6e-121; Fig. 3).
Conclusion(s): Children with obesity show differences in both inflammatory cell makeup (i.e., proportions) and inflammatory gene expression within the CD4+ Tcm cell population. Additionally, obesity severity as measured by BMI correlates with increases in CD4+ Tcm cell proportion. These findings align with prior work illustrating alterations in CD4+ T cell physiology and IL7R overexpression in the obese state, adding novel transcriptomic analysis at the single-cell level in children.
Fig 1. Mean CD4+ Tcm cell proportions across 12 individuals with obesity and 12 controls
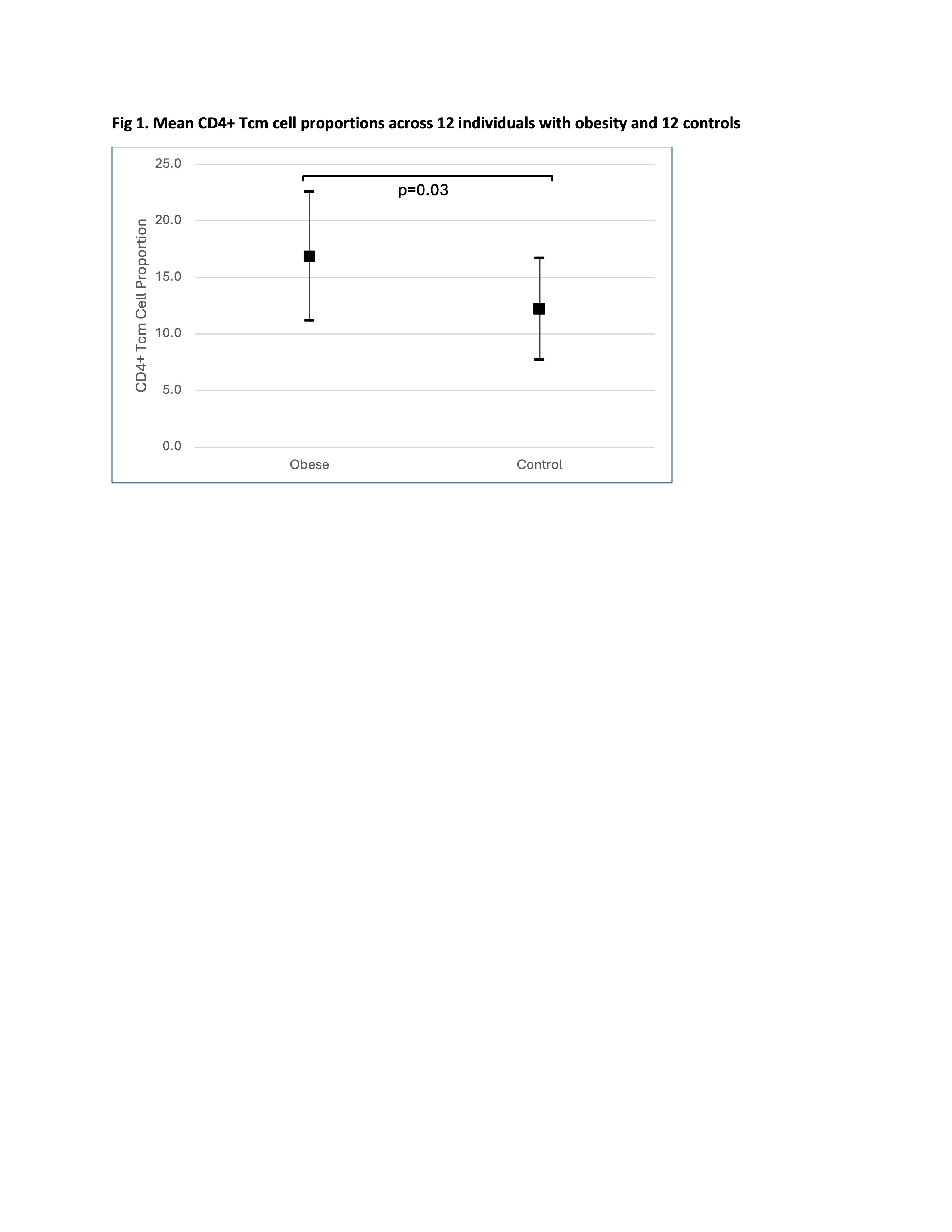
Fig 2. Correlation between body mass index and CD4+ Tcm cell proportion
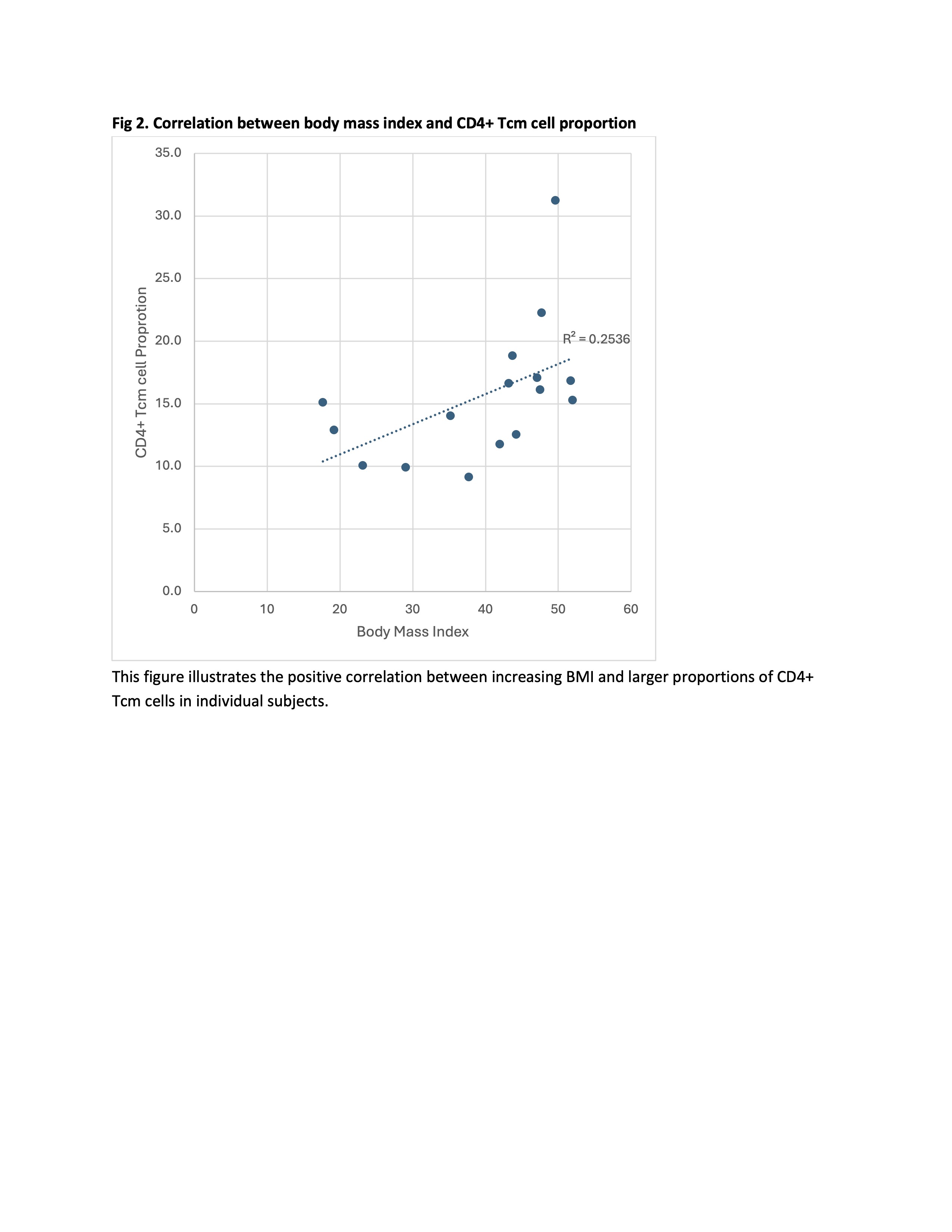 This figure illustrates the positive correlation between increasing BMI and larger proportions of CD4+ Tcm cells in individual subjects.
This figure illustrates the positive correlation between increasing BMI and larger proportions of CD4+ Tcm cells in individual subjects.Fig. 3. IL7R expression level in CD4+ Tcm cells across weight groups
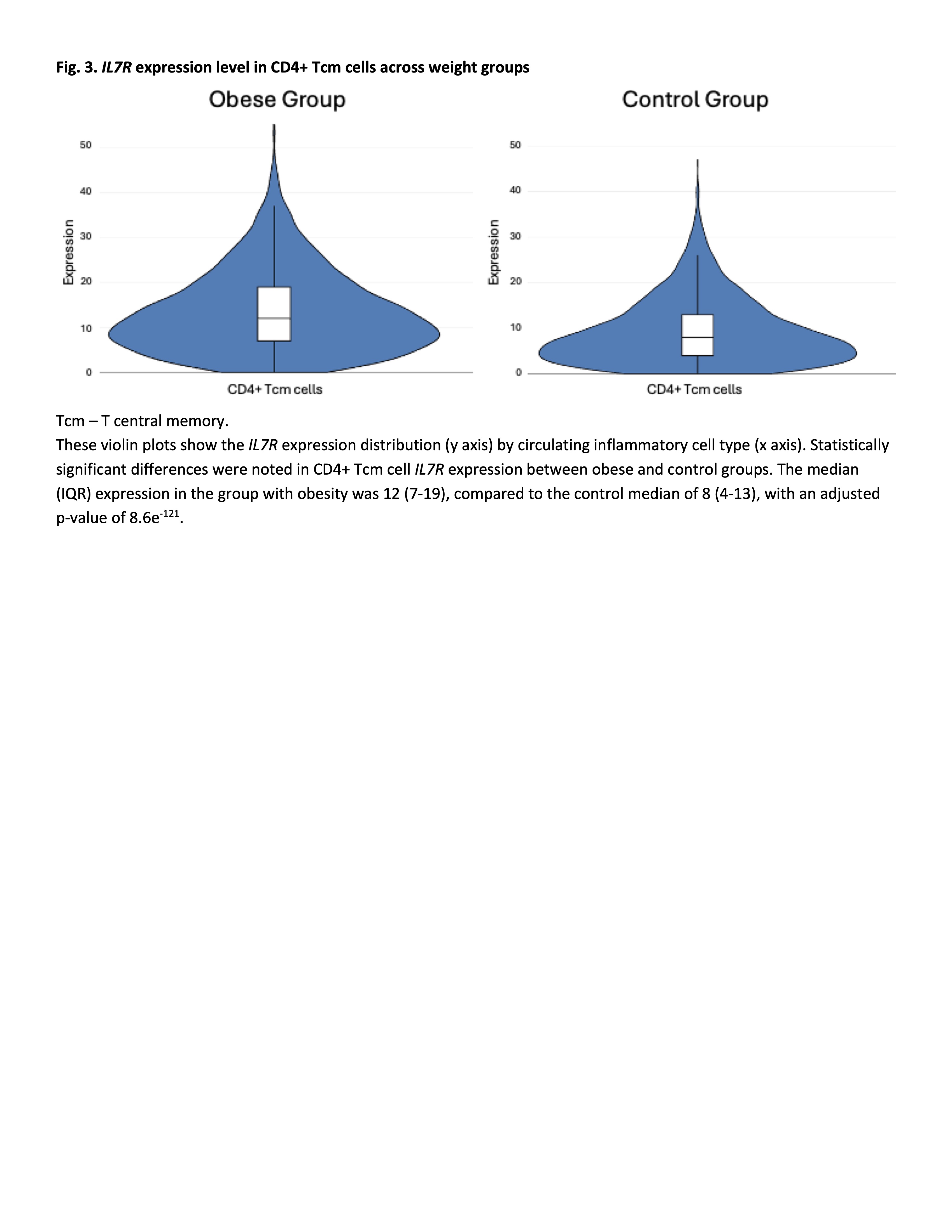 Tcm – T central memory.
Tcm – T central memory.These violin plots show the IL7R expression distribution (y axis) by circulating inflammatory cell type (x axis). Statistically significant differences were noted in CD4+ Tcm cell IL7R expression between obese and control groups. The median (IQR) expression in the group with obesity was 12 (7-19), compared to the control median of 8 (4-13), with an adjusted p-value of 8.6e-121.

