Neonatal Pulmonology - Basic/Translational Science 1
Session: Neonatal Pulmonology - Basic/Translational Science 1
210 - IC100: A Humanized Therapeutic Monoclonal Anti-ASC Antibody Alleviates BPD in Neonatal Mice
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 210.5252
Noor Mehandi, University of Miami Miller School of Medicine, Miami, FL, United States; Rubi Romero Garcia, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Huijun Yuan, University of Miami Medical Center, MIAMI, FL, United States; Shaoyi Chen, University of Miami, Miller school of Medicine, Miami, FL, United States; Matthew Duncan, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Juan Pablo de Rivero Vaccari, University of Miami, Miami, FL, United States; Robert W. Keane, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Merline Benny, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Augusto Schmidt, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Karen C. Young, Division of Neonatology, Department of Pediatrics, Leonard M. Miller School of Medicine, University of Miami, Miami, FL, United States; Shu Wu, University of Miami School of Medicine, Miami, FL, United States

Noor Mehandi, MD
Fellow Physician
University of Miami Miller School of Medicine
Miami, Florida, United States
Presenting Author(s)
Background: Bronchopulmonary dysplasia is one of the common morbidities affecting extremely premature infants. The activation of the inflammasome cascade is implicated in the development of BPD. Apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) is pivotal in inflammasome assembly.
Objective: This study is designed to evaluate:
1. if hyperoxia induces ASC speck formation,
2. test the efficacy of a humanized monoclonal antibody, IC100, directed against ASC, in the treatment of BPD in hyperoxia-exposed neonatal mice.
Design/Methods: 1. To test ASC speck formation in the lung: ASC-citrine reporter mice expressing ASC fusion protein with a C-terminal Citrine (fluorescent GFP isoform) were exposed to 85% oxygen (O2) from postnatal day (P) 1 to P14 that can induce BPD-like pathology. The lungs were dissected on P14 to examine ASC specks.
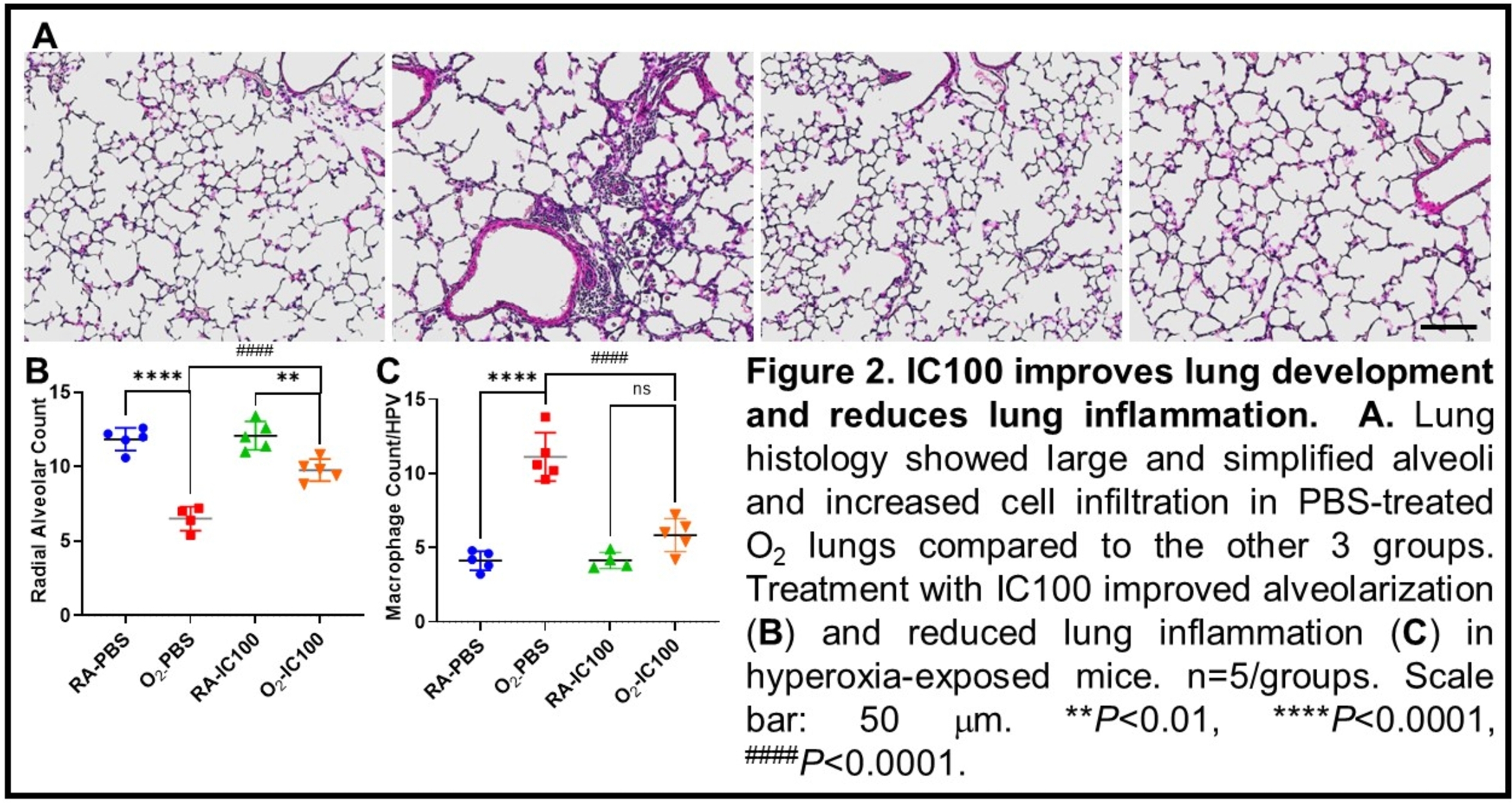
2. To assess the effects of IC100: Newborn mice (C57BL/6J) were randomized to the following groups: room air (RA) with placebo (PBS) (RA-PBS), RA + IC100 intraperitoneal injection (RA-IC100), 85% O2 with PBS (O2-PBS), 85% O2 + IC100 intraperitoneal injection (O2-IC100). The mice were grown in RA or 85% O2 from P1 to P14 as per their assignment. Lungs were dissected for histological evaluation. Lung structures were assessed by radial alveolar count (RAC). Lung inflammation was assessed by counting macrophage infiltration in the alveolar airspace.
Data were presented as mean ± SD and analyzed by ANOVA.
Results: As demonstrated in Figure 1, hyperoxia exposure induced ASC specks in alveolar infiltrated cells, suggesting ASC activation.
As shown in Figure 2, the O2-PBS group had lower RAC than the RA-PBS group. However, the O2-IC100 group had higher RAC than the O2-PBS group, suggesting IC100 improves alveolarization under hyperoxia. The O2-PBS group also had higher infiltrated cells in the alveolar airspaces than the RA-PBS group. While the O2-IC100 group had fewer infiltrated cells in alveolar airspaces than the O2-PBS group, indicating IC100 reduce hyperoxia-induced lung inflammation.
Conclusion(s): Our results demonstrate that hyperoxia activates ASC in the neonatal lungs. Inhibition of ASC activity by IC100 improves alveolar development and reduces lung inflammation in hyperoxia-exposed neonatal lungs. Thus, inhibition of ASC may be beneficial for preventing or treating hyperoxia-induced BPD in preterm infants.
Fig 1: Hyperoxia induces ASC specks in the lung
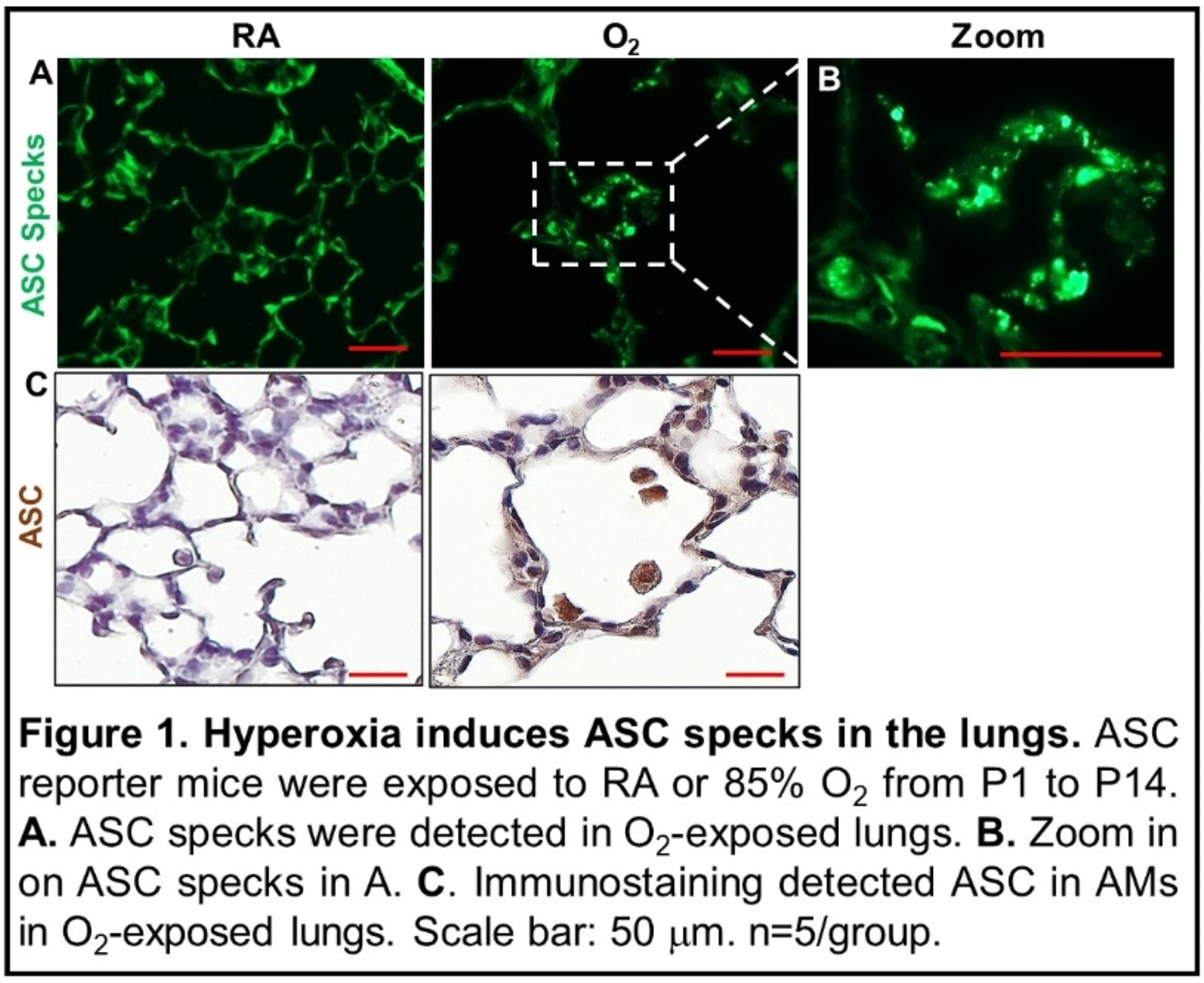
Fig 2: IC100 Improves lung development and reduced lung inflammation