Neonatal Quality Improvement 3
Session: Neonatal Quality Improvement 3
084 - Five years of the Tiny Baby QI Initiative: A “unit within a unit” in a tertiary-level, community NICU.
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 84.5039
Rebecca Sherlock, other, Whistler, BC, Canada

Rebecca Sherlock, MD (she/her/hers)
Neonatologist
other
Whistler, British Columbia, Canada
Presenting Author(s)
Background: Increasing numbers of extremely preterm and growth-restricted infants necessitated a QI initiative addressing care of the tiniest infants in our tertiary-level, community hospital NICU.
Objective: To improve survival and short-term neonatal outcomes by implementing a standardized care approach to inborn infants born < 28 weeks GA +/- < 1000g birthweight.
Design/Methods: A multidisciplinary committee developed a comprehensive care strategy for inborn infants born < 28 weeks GA a+/- < 1000g. Evidence-based practices identified from the literature and and conversations with experts included respiratory support, neuroprotection, VAP avoidance, fluid management, minimal handling, antibiotic stewardship and developmentally supportive care. Pre-implementation (2018-2019; 12 months, n=37) and two PDSA cycles (Cycle 1 - 22 months, n=60 and Cycle 2 - 38 months, n=119, respectively) have been completed. Population characteristics and NICU outcomes were compared.
Results: Median GA decreased from 26 to 25 weeks and the incidence of growth restriction increased over the three eras studied (Table 1). Delayed cord clamping increased significantly over the time periods studied. The use of jet ventilation increased over time and jet ventilation was initiated by 6 hours of life in 67.5% of infants in Cycle 2.
Overall survival improved between Cycle 1 and 2 (Table 2). Of note, survival in infants < 26 weeks GA improved steadily over the three eras.
Short-term NICU outcomes are reported in Table 3. One infant with severe neurologic injury (sNI) was diagnosed with periventricular leukomalacia on day of life one in Cycle 2. With this infant removed, the incidence of sNI in Cycle 2 was 6.25%. CLD rates using various criteria and timing demonstrate stable incidences, regardless of definition. Necrotizing enterocolitis/ SIP increased slightly over the time periods studied (2.7% vs 3.3% vs 5.0%). The proportion of infants treated for a PDA remained stable, however, the agent(s) used to treat varied. In the Pre era, 8 of10 infants treated were exposed to Indomethacin, whereas only 2 of 56 were exposed in Cycle 2. Ibuprofen use was highest in Cycle 1 and Tylenol use was highest in Cycle 3. ROP requiring treatment remained relatively stable (15.5 vs 16.1%).
Conclusion(s): Survival and most short-term outcomes remained stable or improved after the implementation of a standardized program of care in a community-based Level 3 NICU using QI methodology, despite increasing numbers of growth restricted and extremely preterm infants over time. The increase in SIP will have to be examined further.
Table 1. Baseline characteristics of infants born <28 weeks GA and/or <1000g and treated in the Tiny Baby Initiative.
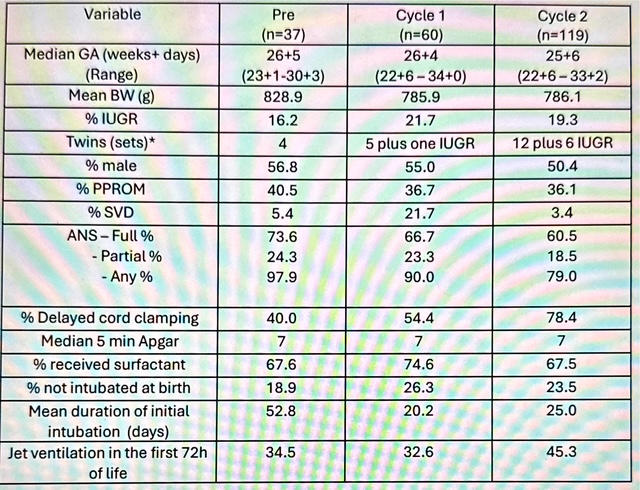 *Number reported reflects full sets of twins plus infants of twin pairs meeting criteria for inclusion in the Tiny Baby Initiative.
*Number reported reflects full sets of twins plus infants of twin pairs meeting criteria for inclusion in the Tiny Baby Initiative. Table 2. Survival by gestational age and cohort for infants treated in the Tiny Baby Initiative.
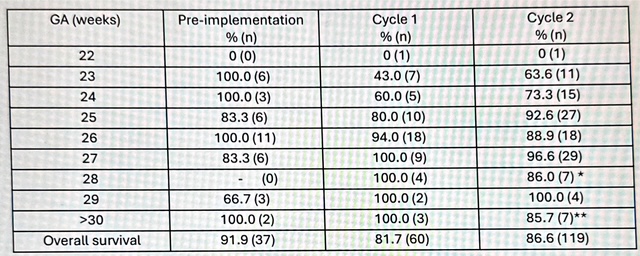 *the one infant that died in this was 600g severely growth restricted
*the one infant that died in this was 600g severely growth restricted **the one infant that died was 500g and 30 weeks GA
Table 3. NICU outcomes for infants treated in the Tiny Baby Initiative.
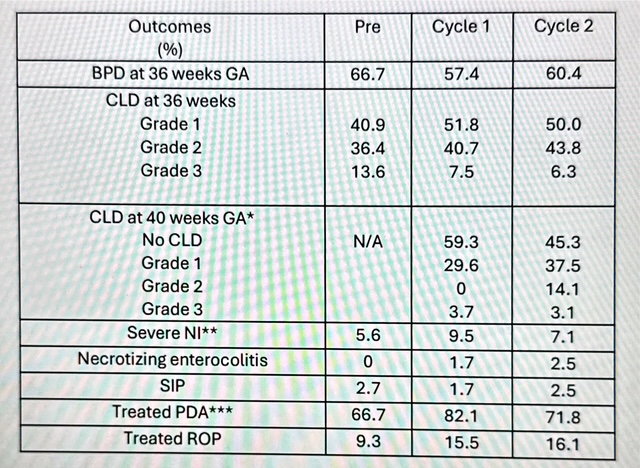 *of those that had CLD at 36 weeks GA
*of those that had CLD at 36 weeks GA **sNI defined as Grade 3 or 4 intraventricular hemorrhage or periventricular leukomalacia
*** of those diagnosed with a PDA, what percentage received treatment


