Back
Background: In single center studies, 27-74% of hospitalized children are missing ≥1 non-influenza vaccine. Despite this, vaccines are administered nationally in < 2% of hospitalizations, with considerable variability in hospital rates of vaccine delivery ranging from 1-45% of visits.
Objective: Our objective was to explore the key determinants for vaccine delivery in a sample of 11 US children’s hospitals.
Design/Methods: We used the Pediatric Health Information System database to identify children’s hospitals in the Pediatric Research in Inpatient Setting network who were high performers (n=5), defined as being in the top tertile of vaccine administration, and low performers (n=5), defined as being in the bottom tertile. The hospital affiliated with the lead investigator was also included. We recruited pediatric nurses, pharmacists, and hospitalists at each of the 11 sites to participate in qualitative semi-structured interviews. Interview questions were based on the Consolidated Framework for Implementation Research (CFIR) and focused on current inpatient vaccine delivery practices, barriers to inpatient vaccine delivery, and domains within the CFIR (Figure 1). Interviews were conducted via telephone or Zoom, recorded and transcribed. Two reviewers independently coded transcripts using thematic analysis with a third reviewer to resolve discrepancies as needed.
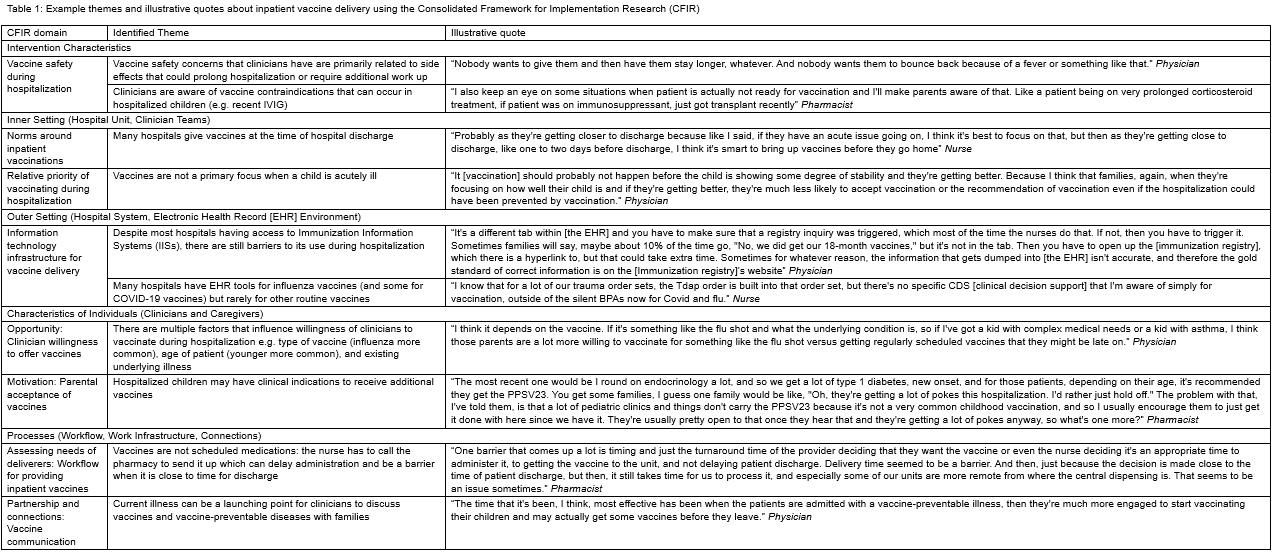
Results: We conducted 30 interviews of 8 hospitalists, 10 nurses, and 12 pharmacists. There were several themes articulated by participants as key determinants for inpatient vaccine delivery (Table 1). Some examples include: 1) Timing during hospitalization: though it was common for all participants across clinician types to screen for vaccine status on admission, delivery of needed vaccines happened predominately at discharge, which at times jeopardized vaccine delivery; 2) Access to care: vaccinating during hospitalization was particularly important when children had difficulty accessing primary care or when traditional healthcare services were disrupted, e.g. the COVID-19 pandemic; 3) Workflow: vaccine-related workflow for inpatient clinicians is often not standardized and lacks ownership.
Conclusion(s): Despite variability in vaccine administration across children’s hospitals, there are many common barriers and determinants. Solutions that address these barriers and meet the needs of multidisciplinary clinicians are essential to improve inpatient vaccine delivery.
Figure 1: Consolidated Framework for Implementation Research (CFIR) for Inpatient Vaccine Delivery
.png)
Table 1: Example themes and illustrative quotes about inpatient vaccine delivery using the Consolidated Framework for Implementation Research (CFIR)
Immunizations/Delivery 2
Session: Immunizations/Delivery 2
686 - Key determinants for inpatient vaccine delivery at 11 US Children’s Hospitals
Friday, April 25, 2025
5:30pm – 7:45pm HST
Mersine Bryan, University of Washington School of Medicine, Seattlle, WA, United States; Alexandra J. Mihalek, CHOC Children's Hospital of Orange County, Long Beach, CA, United States; Richard Torres, Seattle Children's, Seattle, WA, United States; Sanyukta Desai, Dell Children's Medical Center of Central Texas, Austin, TX, United States; Susan Wu, Children's Hospital Los Angeles/USC Keck School of Medicine, Los Angeles, CA, United States; Erin Avondet, University of Utah School of Medicine, Salt Lake City, UT, United States; Rachel Solstad, University of Utah School of Medicine, Salt Lake City, UT, United States; Vanessa McFadden, Medical College of Wisconsin, MIlwaukee, WI, United States; Rena Kasick, Nationwide Children's Hospital, Columbus, OH, United States; Sumeet Banker, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States; Daniel C. Williams, MUSC, Mount Pleasant, SC, United States; Sunita Hemani, Children's Healthcare of Atlanta, Atlanta, GA, United States; Catherine Forster, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Douglas J.. Opel, Seattle Children's Research Institute, Seattle, WA, United States
- MB
Mersine Bryan, MD, MPH (she/her/hers)
Assistant Professor
University of Washington School of Medicine
Seattlle, Washington, United States
Presenting Author(s)
Background: In single center studies, 27-74% of hospitalized children are missing ≥1 non-influenza vaccine. Despite this, vaccines are administered nationally in < 2% of hospitalizations, with considerable variability in hospital rates of vaccine delivery ranging from 1-45% of visits.
Objective: Our objective was to explore the key determinants for vaccine delivery in a sample of 11 US children’s hospitals.
Design/Methods: We used the Pediatric Health Information System database to identify children’s hospitals in the Pediatric Research in Inpatient Setting network who were high performers (n=5), defined as being in the top tertile of vaccine administration, and low performers (n=5), defined as being in the bottom tertile. The hospital affiliated with the lead investigator was also included. We recruited pediatric nurses, pharmacists, and hospitalists at each of the 11 sites to participate in qualitative semi-structured interviews. Interview questions were based on the Consolidated Framework for Implementation Research (CFIR) and focused on current inpatient vaccine delivery practices, barriers to inpatient vaccine delivery, and domains within the CFIR (Figure 1). Interviews were conducted via telephone or Zoom, recorded and transcribed. Two reviewers independently coded transcripts using thematic analysis with a third reviewer to resolve discrepancies as needed.
Results: We conducted 30 interviews of 8 hospitalists, 10 nurses, and 12 pharmacists. There were several themes articulated by participants as key determinants for inpatient vaccine delivery (Table 1). Some examples include: 1) Timing during hospitalization: though it was common for all participants across clinician types to screen for vaccine status on admission, delivery of needed vaccines happened predominately at discharge, which at times jeopardized vaccine delivery; 2) Access to care: vaccinating during hospitalization was particularly important when children had difficulty accessing primary care or when traditional healthcare services were disrupted, e.g. the COVID-19 pandemic; 3) Workflow: vaccine-related workflow for inpatient clinicians is often not standardized and lacks ownership.
Conclusion(s): Despite variability in vaccine administration across children’s hospitals, there are many common barriers and determinants. Solutions that address these barriers and meet the needs of multidisciplinary clinicians are essential to improve inpatient vaccine delivery.
Figure 1: Consolidated Framework for Implementation Research (CFIR) for Inpatient Vaccine Delivery
.png)
Table 1: Example themes and illustrative quotes about inpatient vaccine delivery using the Consolidated Framework for Implementation Research (CFIR)


