Pediatric Therapeutics and Pharmacology
Session: Pediatric Therapeutics and Pharmacology
818 - Pharmacokinetics and breastmilk transfer to infants of subcutaneous extended-release buprenorphine and N-methyl-2-pyrrolidone (NMP) component in lactating individuals with opioid use disorder
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 818.6126
Elisha M. Wachman, Boston University School of Medicine, Boston, MA, United States; Xin Xie, Boston Medical Center, Boston, MA, United States; Kelley Saia, Boston University School of Medicine, Lynnfield, MA, United States; Davida Schiff, Massachusetts General Hospital, Boston, MA, United States; Jeremiah Momper, University of California, San Diego, La Jolla, CA, United States

Elisha M. Wachman, MD (she/her/hers)
Professor of Pediatrics
Boston Medical Center
Boston, Massachusetts, United States
Presenting Author(s)
Background: Extended-release buprenorphine (XR BUP) is a commonly utilized medication for non-pregnant individuals with opioid use disorder (OUD) and some prefer it compared to daily sublingual buprenorphine (SL BUP). N-methyl-2-pyrrolidone (NMP), an excipient of monthly XR BUP formulations that aides in the slow release, has the potential for developmental toxicity based on animal studies, with no previous human studies measuring NMP plasma levels after XR BUP. Further, no information is available on the pharmacokinetics or breastmilk transfer in lactating individuals receiving XR BUP.
Objective: To evaluate maternal, breastmilk, and infant concentrations of buprenorphine (BUP) and NMP in lactating individuals on XR BUP and their infants.
Design/Methods: Lactating individuals between 0-6 months postpartum receiving XR BUP were enrolled. Paired maternal plasma, infant plasma, and breast milk samples were collected at up to 3 time points. All samples were analyzed for BUP and NMP concentrations using a validated liquid chromatography tandem mass spectrometry assay. The relative infant dose (RID) was estimated by using an average milk intake of 150 mL/kg/day and observed breastmilk concentrations.
Results: A total of 3 lactating individuals were enrolled, all receiving 300mg of XR BUP subcutaneously every month. Samples were taken at a mean of 16.5 (SD 5.4) weeks postpartum and a mean of 16.5 days after the last dose of XR BUP. A total of 9 maternal plasma, 6 infant plasma, and 5 breastmilk samples were collected (Table 1). Maternal plasma concentrations (mean 6.0 ng/mL, SD 1.6) were similar to exposures previously reported in non-pregnant individuals on XR BUP and postpartum individual on SL BUP. Breastmilk concentrations of BUP (mean 8.9 ng/mL, SD 6.6) were similar to previously reported SL BUP with a RID of 0.4%.(Table 2) BUP was below the lower limit of quantitation ( < 0.5 ng/mL) for all infant plasma samples. NMP was detectable in maternal plasma (mean 5.43μg/mL, SD 4.56) and breastmilk (mean 3.83 μg/mL, SD 5.07) from samples measured between 1-5 hours after subcutaneous dosing, with levels dropping off when measured between 5-35 days after dosing. No NMP was detected in the infant plasma samples.(Figure 1)
Conclusion(s): BUP is present in low levels in breastmilk of lactating individuals receiving XR BUP resulting in a low RID. However, as NMP – a known reproductive and developmental toxicant – passes into breastmilk, individuals receiving XR BUP should be cautioned if breastfeeding. Additional data is needed about XR BUP and lactation before definitive conclusions can be made.
Table 1
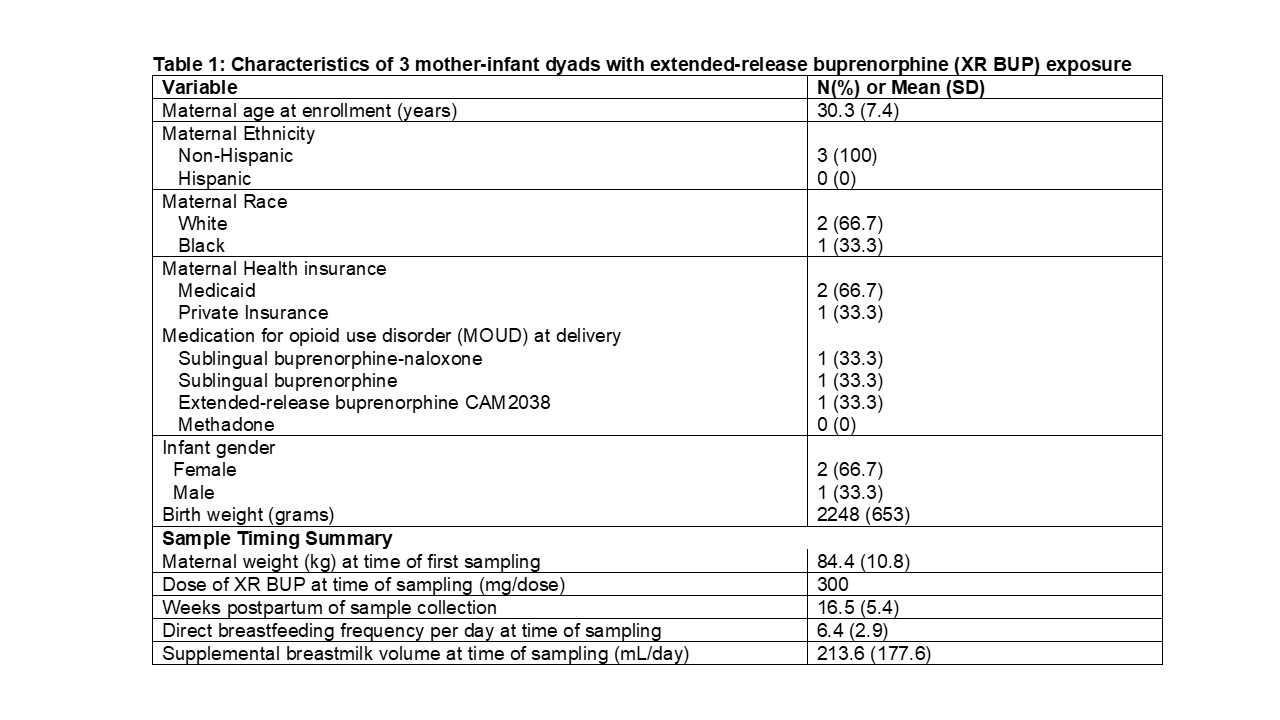
Table 2
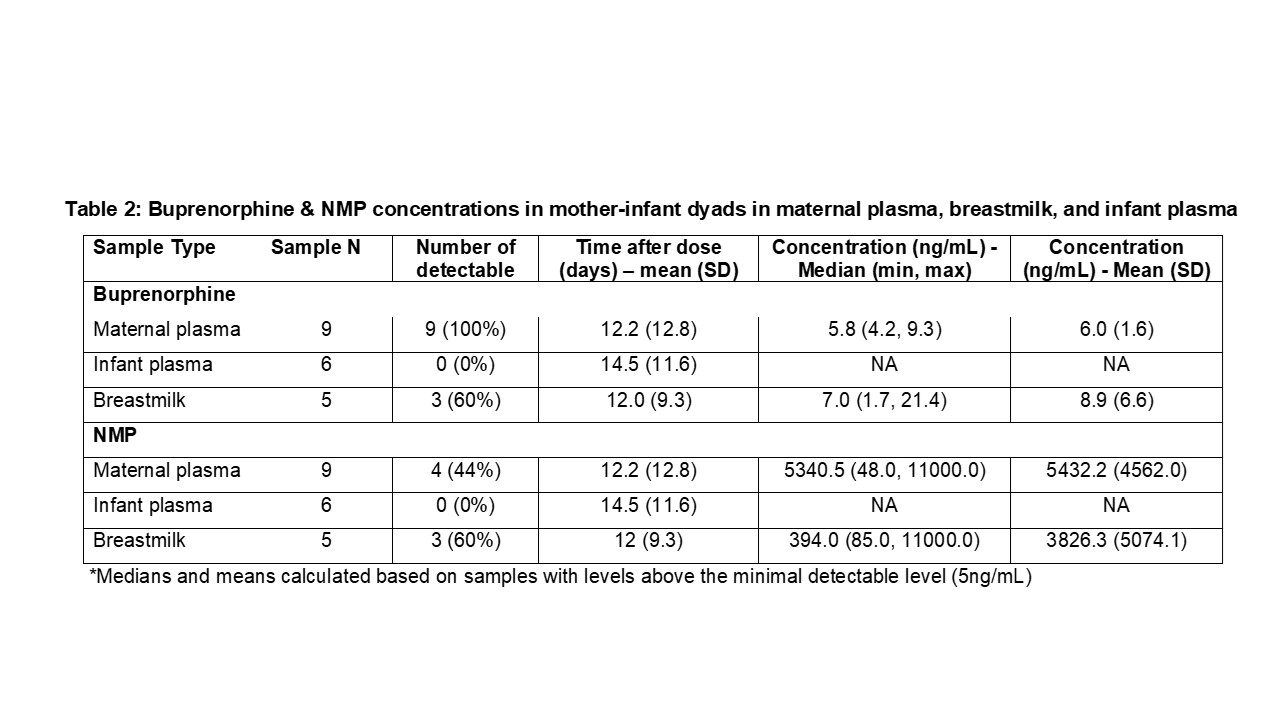
Figure 1
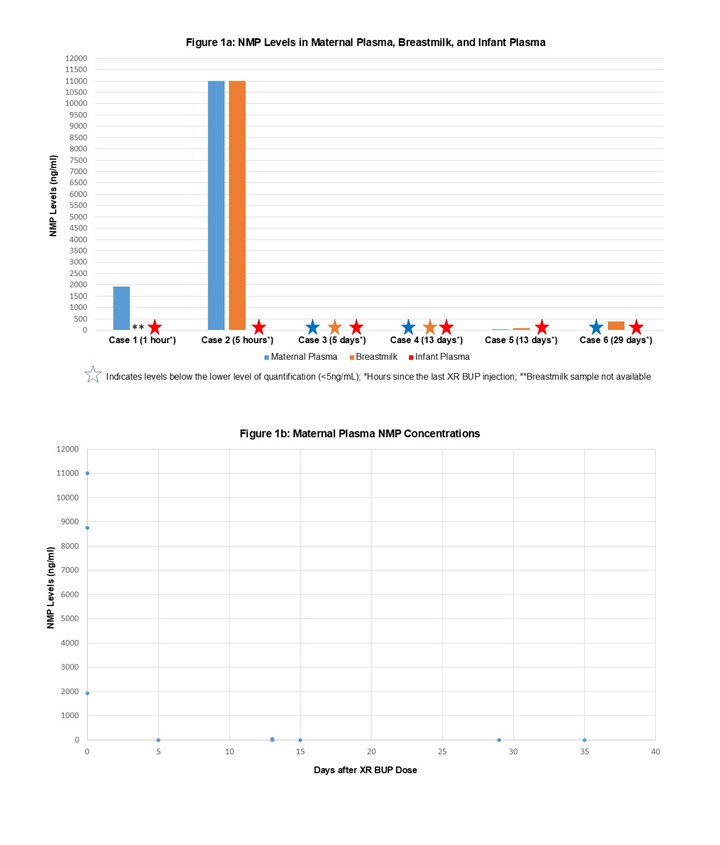
Table 1

Table 2

Figure 1


