Neonatal Neurology 3
Session: Neonatal Neurology 3
368 - Dexmedetomidine Use in Infants undergoing Cooling due to Neonatal Encephalopathy (DICE trial): A Multi-Center, Phase II Controlled Randomized Safety and Pharmacokinetic Study
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 368.4516
Mariana Baserga, University of Utah School of Medicine, Salt lake City, UT, United States; Timothy M. Bahr, University of Utah, Provo, UT, United States; Carrie Rau, University of Utah School of Medicine, Salt Lake City, UT, United States; Tara L. DuPont, University of Utah, Salt Lake City, UT, United States; Betsy Ostrander, University of Utah School of Medicine, Salt Lake City, UT, United States; Michelle Yang, University of Utah School of Medicine, Salt Lake City, UT, United States; Anastasiya Mankouski, Intermountain Health, Salt Lake City, UT, United States; Kevin Watt, University of Utah School of Medicine, Salt Lake City, UT, United States
- MB
Mariana Baserga, MD (she/her/hers)
Professor of Pediatrics
University of Utah School of Medicine
Salt Lake City, Utah, United States
Presenting Author(s)
Background: Despite therapeutic hypothermia (TH), up to 30% of infants with neonatal hypoxic-ischemic encephalopathy (NE) either die or have motor and cognitive dysfunction. Studies suggest that neurodevelopmental (ND) outcomes may be improved by treating pain and agitation associated with TH. Dexmedetomidine (DEX) is an α2-adrenergic receptor agonist that may be a better alternative to morphine as it provides sedation and analgesia but does not suppress ventilation. Importantly, there is increasing evidence that DEX has neuroprotective properties in animal models of HIE. Despite limited data on pharmacokinetics (PK), safety, and efficacy in this population DEX is increasingly administered in many centers during TH.
Objective: To assess safety, outcomes and PK of DEX administered to infants with moderate to severe NE undergoing TH.
Design/Methods: Phase II multicenter, randomized, safety and PK trial. Infants with moderate or severe NE were randomized to receive either DEX (1 μg/kg loading dose; up to 0.5 μg/kg/h continuous IV) or morphine (up to 0.05 mg/kg/dose q4 hours IV or continuous IV dose up to 0.01 mg/kg/hr) during TH. Dosing was adjusted using the Neonatal Pain, Agitation, and Sedation Scale (N-PASS) scores. Adverse events (AE) were collected during TH. Subjects had 2 opportunistic PK blood samples at the time of routine laboratories. DEX population PK was developed using nonlinear mixed effects modeling software (NONMEM). The Assessment of General Movements (GMA) and Hammersmith Infant Neurological Exam (HINE) was used to assess ND at 3 and 9 months of age.
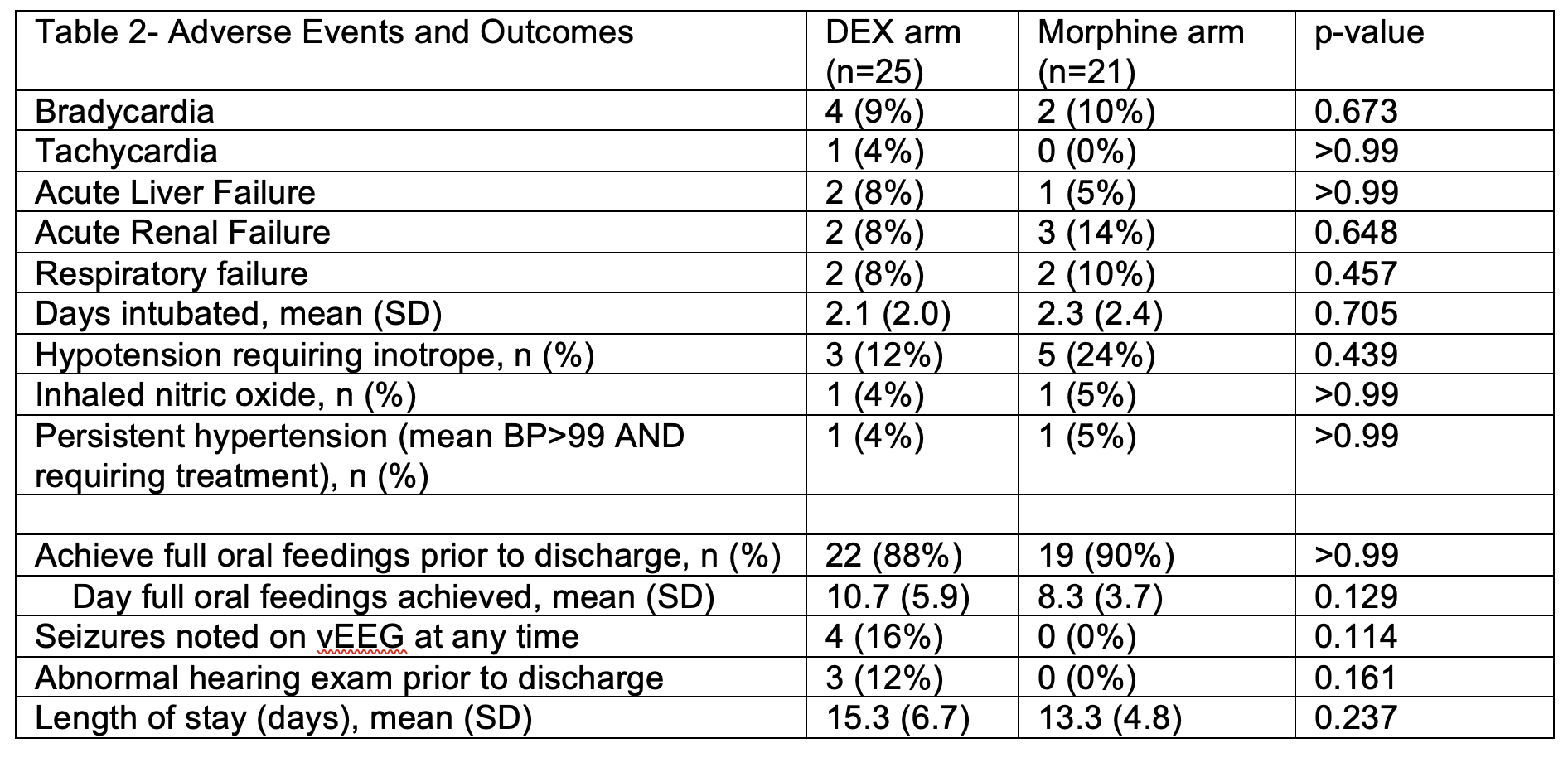
Results: Twenty-five infants randomized to DEX and 21 to morphine. There were no differences in demographic and baseline characteristics between groups (Table 1) and in hospitalization outcomes and distribution of AEs (Table 2). A total of 15 subjects contributed 32 PK samples using a 1-compartment model with first-order elimination analysis. Median clearance and volume of distribution were 0.51 L/kg/h and 0.25 L/kg. At the time of analysis, there were no differences between infants receiving morphine (n=16) versus DEX (n=22) in writhing and fidgety stage GMAs, and 3-month HINE (p>0.5).
Conclusion(s): This safety and PK prospective study comparing DEX to morphine use in infants undergoing TH showed similar incidence of AEs, hospitalization and 3 months' ND outcomes in the 2 groups. DEX clearance and volume of distribution was lower than in non-cooled infants in previous reports. ND follow-up at 9 months is still ongoing. DEX used in the dosing proposed in this trial seems safe. A larger efficacy trial studying neuroprotective benefits of DEX is warranted.
Table 1- Demographics
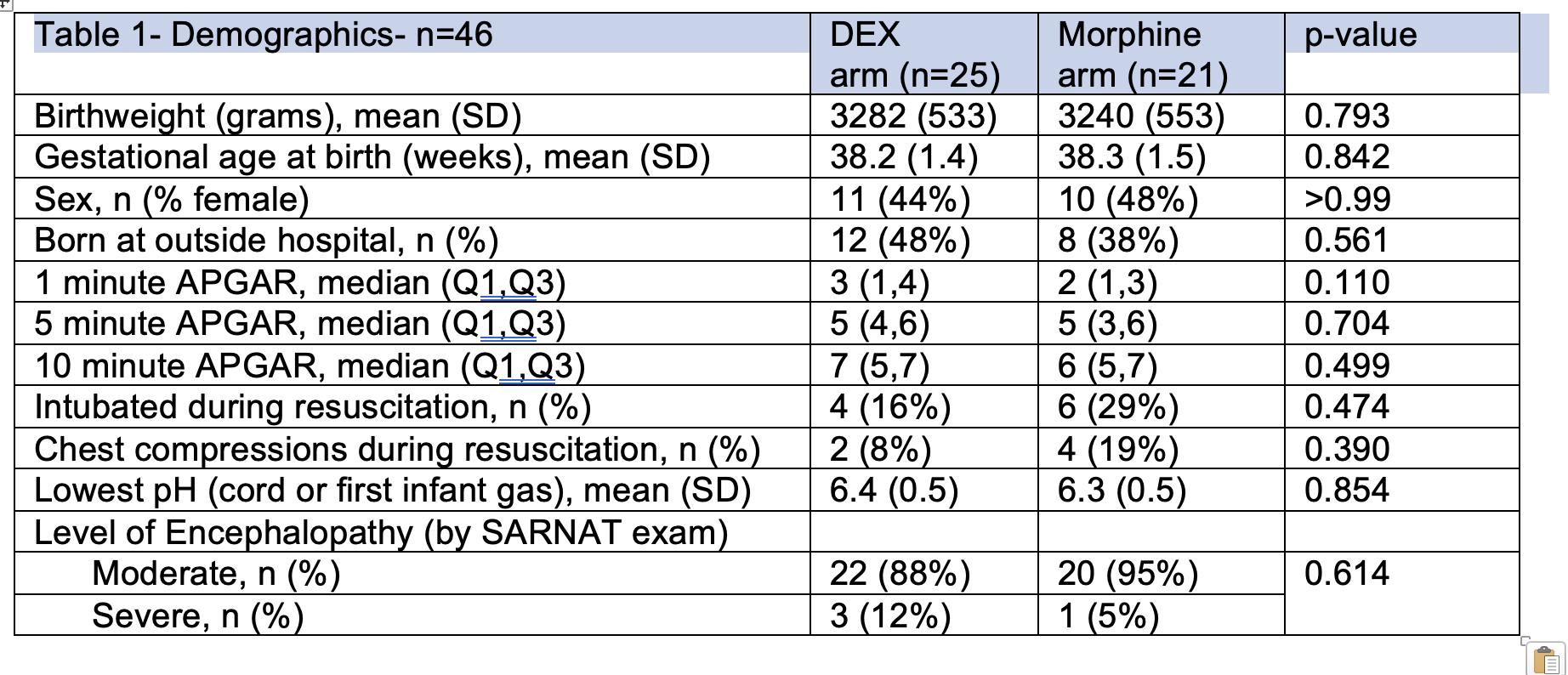
Table 2- Adverse Events and Short term Hospital Outcomes