Pulmonology
Session: Pulmonology
095 - A Path to Equity In Cystic Fibrosis Newborn Screening: CF Foundation Newborn Screening Recommendations
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 95.3775
Meghan E. McGarry, University of Washington School of Medicine, Seattle, WA, United States; Karen S. Raraigh, Johns Hopkins University, Columbia, MD, United States; Marissa Taylor, Cystic Fibrosis Foundation, Bethesda, MD, United States; Sarah Hempstead, Cystic Fibrosis Foundation, Bethesda, MD, United States; Al Faro, Cystic Fibrosis Foundation, Bethesda, MD, United States; Susanna McColley, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Marci K. Sontag, Center for Public Health Innovation, Evergreen, CO, United States

Meghan E. McGarry, MD, MAS, ATSF (she/her/hers)
Associate Professor of Pediatrics
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: Newborn screening (NBS) for cystic fibrosis (CF) should ensure all infants with CF have early diagnosis and treatment, but the current state of CF NBS has highlighted existing disparities in diagnosis, treatment, and outcomes. Infants with CF who are Asian, Black, or Hispanic are more likely to have a false negative NBS. Due to the variation in NBS program algorithms, timely diagnosis is determined by where an infant with CF is born.
Objective: The CF Foundation (CFF) developed a guideline to improve the equity of CF NBS.
Design/Methods: A committee of parents of children with CF, a primary care pediatrician, CF physicians, a genetic counselor, a bioethicist, NBS lab directors, and CFF staff developed six questions to identify the NBS processes that impact equity, sensitivity, and timeliness of CF diagnosis. After systematic reviews, the committee developed and voted on recommendations for CF NBS. Other considerations to achieve timeliness and equity for all infants with CF were discussed.
Results: The committee proposed that NBS achieve a 95% sensitivity or true-positive CF detection rate for across all ancestral groups. Recommendations addressed immunoreactive trypsinogen (IRT), CFTR variant testing, and communication of results (Figure 1, Table 1). There were many considerations for pediatricians (Table 2). Notification of a positive NBS should involve the primary care provider, who likely has built more trust with the family. CF providers should also be notified to facilitate timely evaluation for infants of all races, ethnicities, and ancestries. NBS results should be provided in the family’s native language and be understandable to those with low health literacy. NBS programs should take into consideration that preterm and critically ill infants have elevated IRT levels. Infants with CF born to a birthing parent on CFTR modulator therapy during pregnancy may have a false negative NBS. Infants with a full sibling with CF or whose parents are CF carriers should have a diagnostic evaluation for CF regardless of the NBS result. Pediatricians should know that NBS is a screening test only, that all people with signs or symptoms of CF should have a diagnostic evaluation, regardless of the NBS result, and that CF occurs in people of all races, ethnicities, and ancestries.
Conclusion(s): NBS guidelines with improved care from the medical community are needed to achieve equity for all infants with CF.
Figure 1
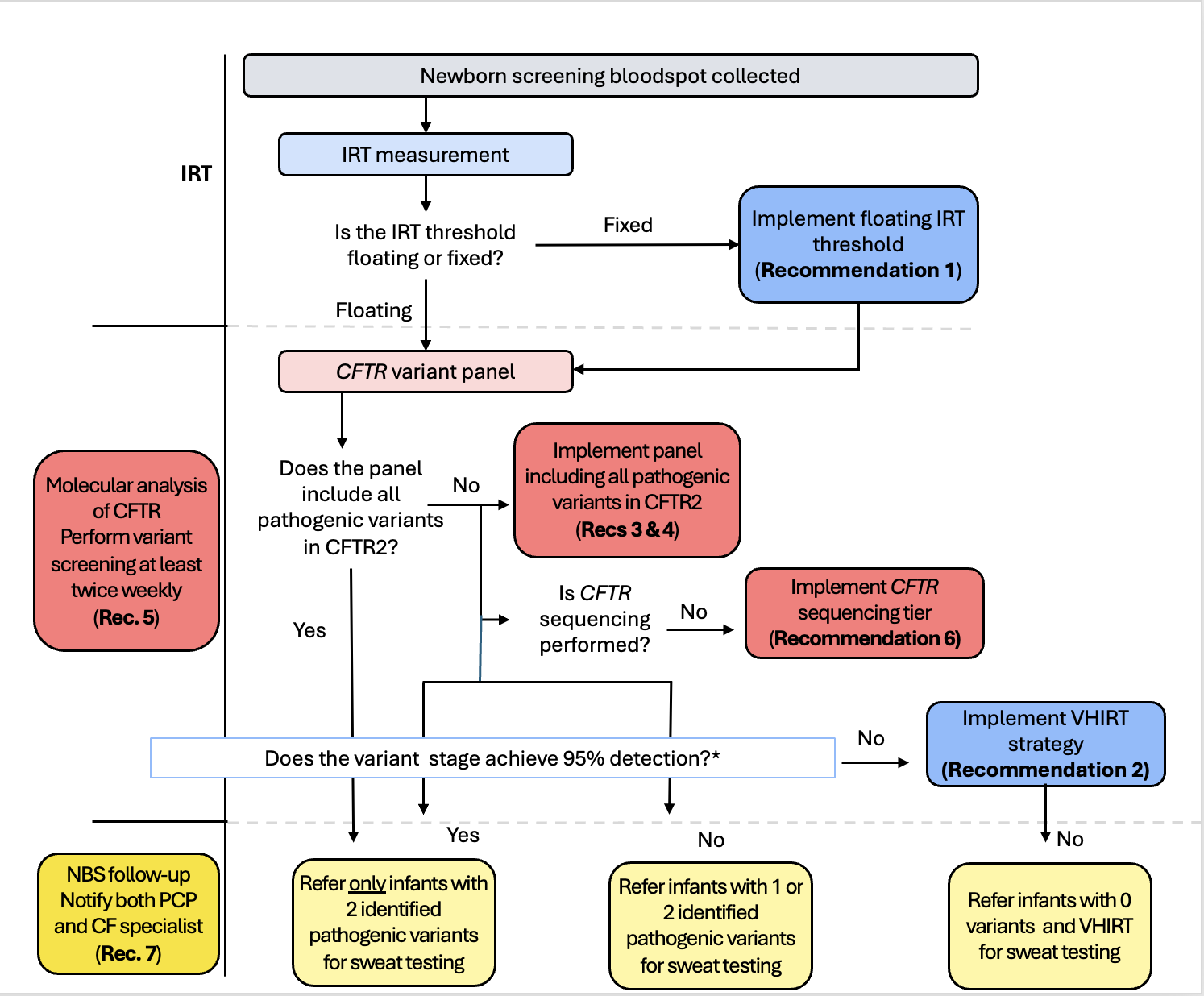 Flowchart of ideal CF NBS algorithm and decision points for implementing change
Flowchart of ideal CF NBS algorithm and decision points for implementing changeTable 1
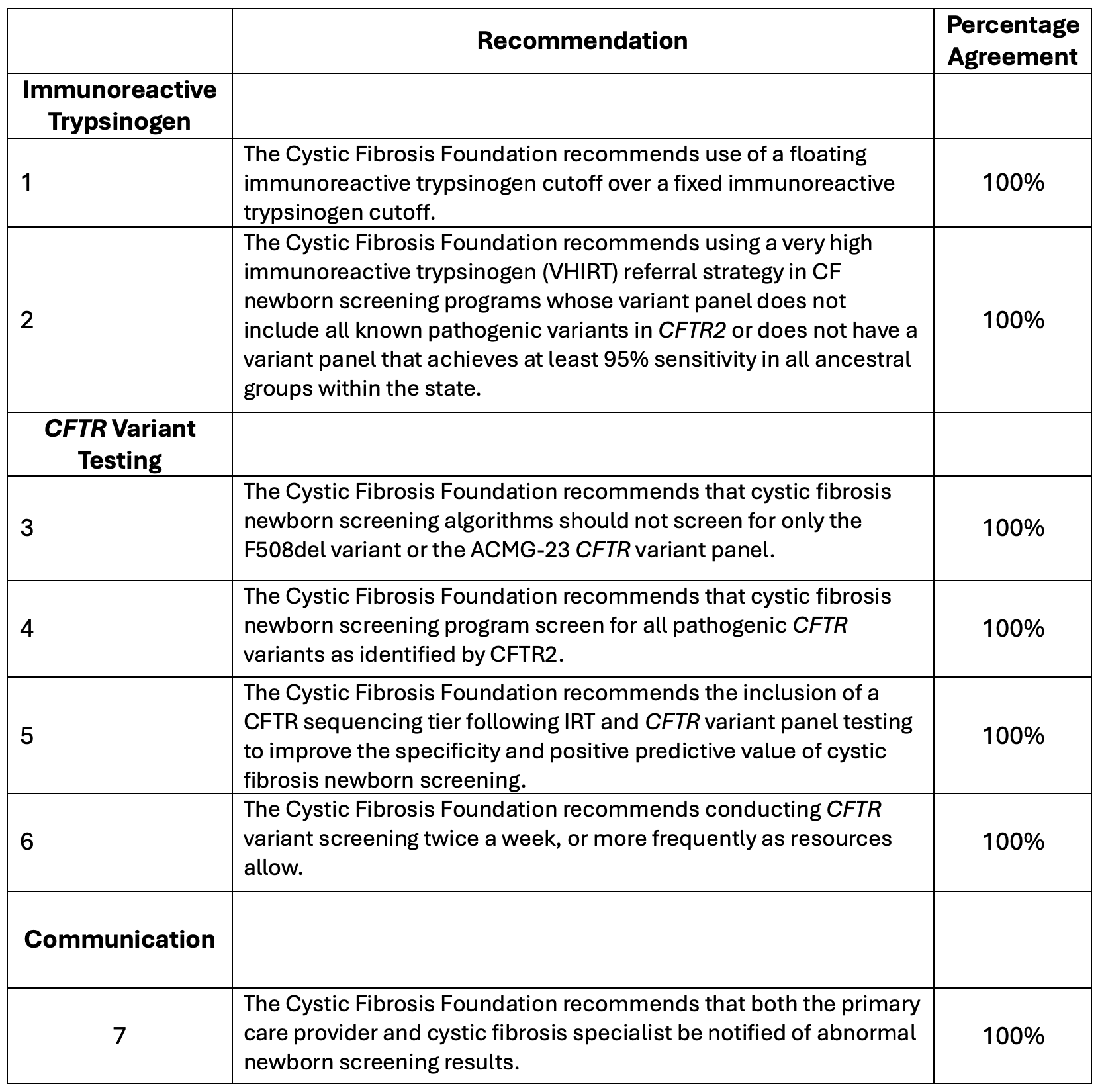 Consensus Recommendation Statements for Cystic Fibrosis Newborn Screening
Consensus Recommendation Statements for Cystic Fibrosis Newborn ScreeningTable 2:
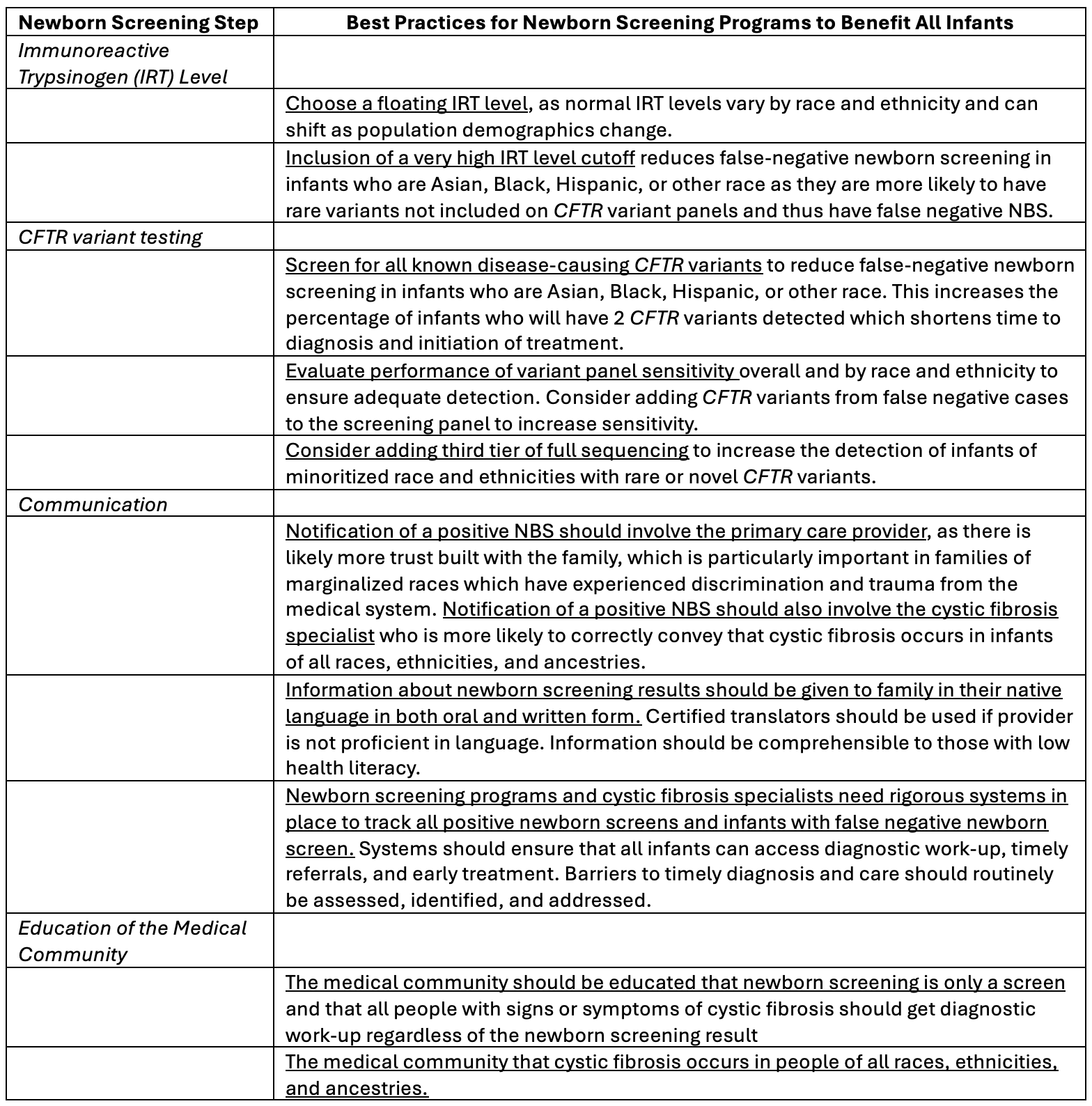 Considerations for Equity in Cystic Fibrosis Newborn Screening
Considerations for Equity in Cystic Fibrosis Newborn Screening
