Neonatal General 3: NICU Practices
Session: Neonatal General 3: NICU Practices
559 - Stability and sterility of caffeine citrate after vial opening for up to 96 hours: an in-vitro study
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 559.6705
Nattapol Rungrojjananon, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Krung Thep, Thailand; Buranee Swatesutipun, Ramathibodi Hospital, Mahidol University, Bangkok, Krung Thep, Thailand; Pracha Nuntnarumit, Faculty of Medicine Ramathibodi Hospital, Bangkok, Krung Thep, Thailand; Chatchay Prempunpong, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Krung Thep, Thailand; Wararat Yangsuk, Mahidol University, Krung Thep, Krung Thep, Thailand; Wararat Chiangjong, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Rachatewi, Krung Thep, Thailand; Apichaya Puangpetch, Mahidol university, Bangkok, Krung Thep, Thailand; Santirhat Prommas, Mahidol university, Bangkok, Krung Thep, Thailand; Pailin Srisuratsiri, Faculty of Science at Sriracha, Sriracha, Chon Buri, Thailand

Nattapol Rungrojjananon (he/him/his)
Doctor
Faculty of Medicine, Ramathibodi Hospital, Mahidol University
Bangkok, Krung Thep, Thailand
Presenting Author(s)
Background: Apnea of prematurity (AOP) is a common problem in daily
practice. Caffeine citrate (CC) is widely used to treat AOP due to its
efficacy and safety. However, its use is limited due to cost concerns in resource-limited countries. The recommended stability of CC after vial opening by the manufacturer is 24 hours at room temperature.
Objective: This study aimed to explore the extended stability and sterility of CC.
Design/Methods: An in-vitro study was performed to determine the stability of caffeine using Liquid chromatography-tandem mass spectrometry (LC-MS/MS). After opening the vial of CC, five syringes containing 0.2 mL of CC were prepared with a sterile technique and stored at 2-8°C for 0, 24, 48, 72, and 96 hours until analysis. We assessed any changes in appearance, precipitation, and pH during this period. Additionally, we confirmed sterility through endotoxin testing using an endotoxin assay kit and aerobic bacterial culture.
Results: None of the caffeine products showed any appearance, pH, or
precipitation changes. The LC-MS/MS analysis indicated that the accuracy of calculated concentrations of caffeine stored for 0, 24, 48, 72, and 96 hours at 2-8°C were between 89.83% and 109.23% by using the reference of US FDA bioanalytical method validation guidance (2018). No bacterial endotoxins were detected at levels exceeding 0.01 EU/mL, the threshold for measurable endotoxin concentration. No bacterial growth was detected in aerobic bacterial cultures.
Conclusion(s): Our study confirms that CC retains its stability and sterility for an extended period of 96 hours when stored at 2-8ºC. This finding could offer a cost-saving opportunity in managing apnea of prematurity in resource-limited settings.
Stability analysis of caffeine citrate over 96 hours after vial opening
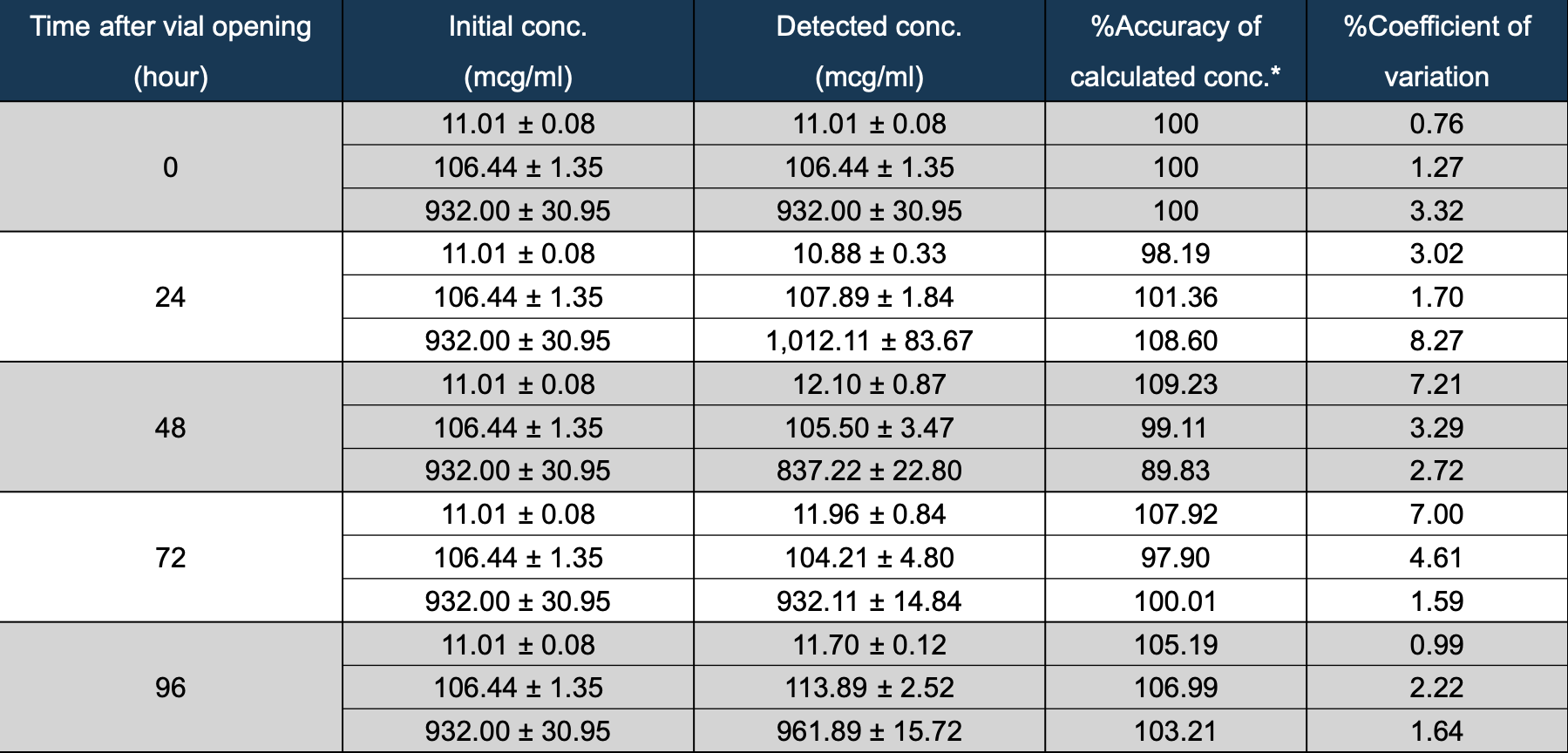 Accuracy of caffeine citrate concentrations stored at 2-8°C for 0, 24, 48,
Accuracy of caffeine citrate concentrations stored at 2-8°C for 0, 24, 48,72, and 96 hours, remained within 89.83% to 109.23%, meeting the
2018 US FDA bioanalytical method validation guidelines.

