Neonatal General 2: Neurology
Session: Neonatal General 2: Neurology
324 - Modifiable Risk Factors for Delirium in NICU Patients: Focus on Medication Use
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 324.5925
Divya Rana, UTHSC, Memphis, TN, United States; Kathryn Martin, University of Tennessee Health Science Center College of Medicine, Memphis, TN, United States; Mark F. Weems, University of Tennessee Health Science Center College of Medicine, Memphis, TN, United States
- DR
Divya Rana, MD (she/her/hers)
Associate Professor
UTHSC
University of Tennessee Health Science Center
Memphis, Tennessee, United States
Presenting Author(s)
Background: Delirium in neonatal intensive care units (NICUs) has significant clinical consequences, yet the modifiable risk factors contributing to its development are not well established.
Objective: This study investigates the relationship between delirium and exposure to commonly used neuromodulating medications, including opioids, benzodiazepines, and alpha agonists, with the goal of identifying modifiable risks.
Design/Methods: A retrospective cohort study was conducted on infants ≥40 weeks post-menstrual age (PMA) admitted to a Level IV NICU between April 1, 2023, and March 31, 2024. All infants had at least one documented Cornell Assessment for Pediatric Delirium (CAPD) score, with a score of ≥9 indicating delirium. CAPD score is administered as standard of care in NICU. Medication exposure was analyzed at the time of the highest recorded CAPD score, specifically focusing on benzodiazepines, opioids, and dexmedetomidine.
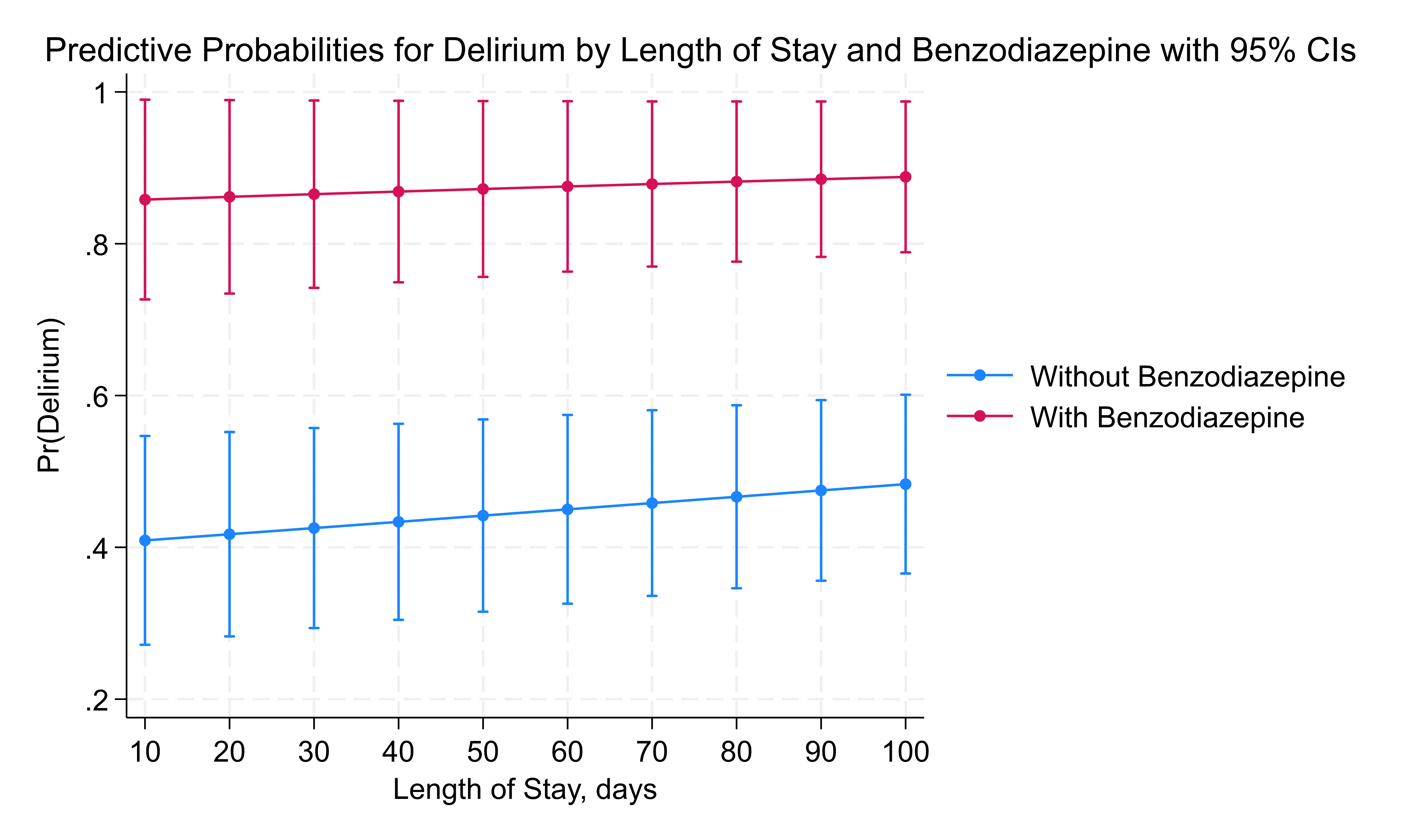
Results: Among 153 infants, 99 (65%) met the diagnostic criteria for delirium. Both the use of opioids and benzodiazepines at the time of high CAPD score were significant risks for delirium, p < 0.01, however dexmedetomidine did not show this risk, p = 0.36. Significantly, more infants in delirium group were treated for iatrogenic withdrawal or agitation issues, 52% versus 22%, p < 0.01. Benzodiazepine exposure was strongly associated with an increased risk of delirium, with infants having 20 times higher odds of developing delirium compared to those not exposed (p = 0.005, CI: 2.5–160.4), figure 1 and for LOS, each additional day in the hospital, the odds of delirium increase by 0.4% (p = 0.013). With benzodiazepine use, the probability of delirium was consistently high, regardless of LOS figure 2. Dexmedetomidine exposure alone did not significantly increase the odds of delirium (p = 0.41). However, when benzodiazepines were combined with dexmedetomidine, the risk of delirium decreased by 86% (p = 0.09, CI: 0.015–1.359), suggesting a potential protective effect, though this finding was only marginally significant.
Conclusion(s): Benzodiazepine use significantly increases the risk of delirium in NICU patients, whereas combined use with dexmedetomidine may reduce this risk. Identifying and managing modifiable risk factors such as medication exposure could be key to reducing the incidence of delirium in this vulnerable population. These findings point to the need for targeted intervention protocols to optimize medication use and minimize delirium risk in NICU settings.
Table 1. Clinical Characteristics and Medication Use for Infants by Delirium
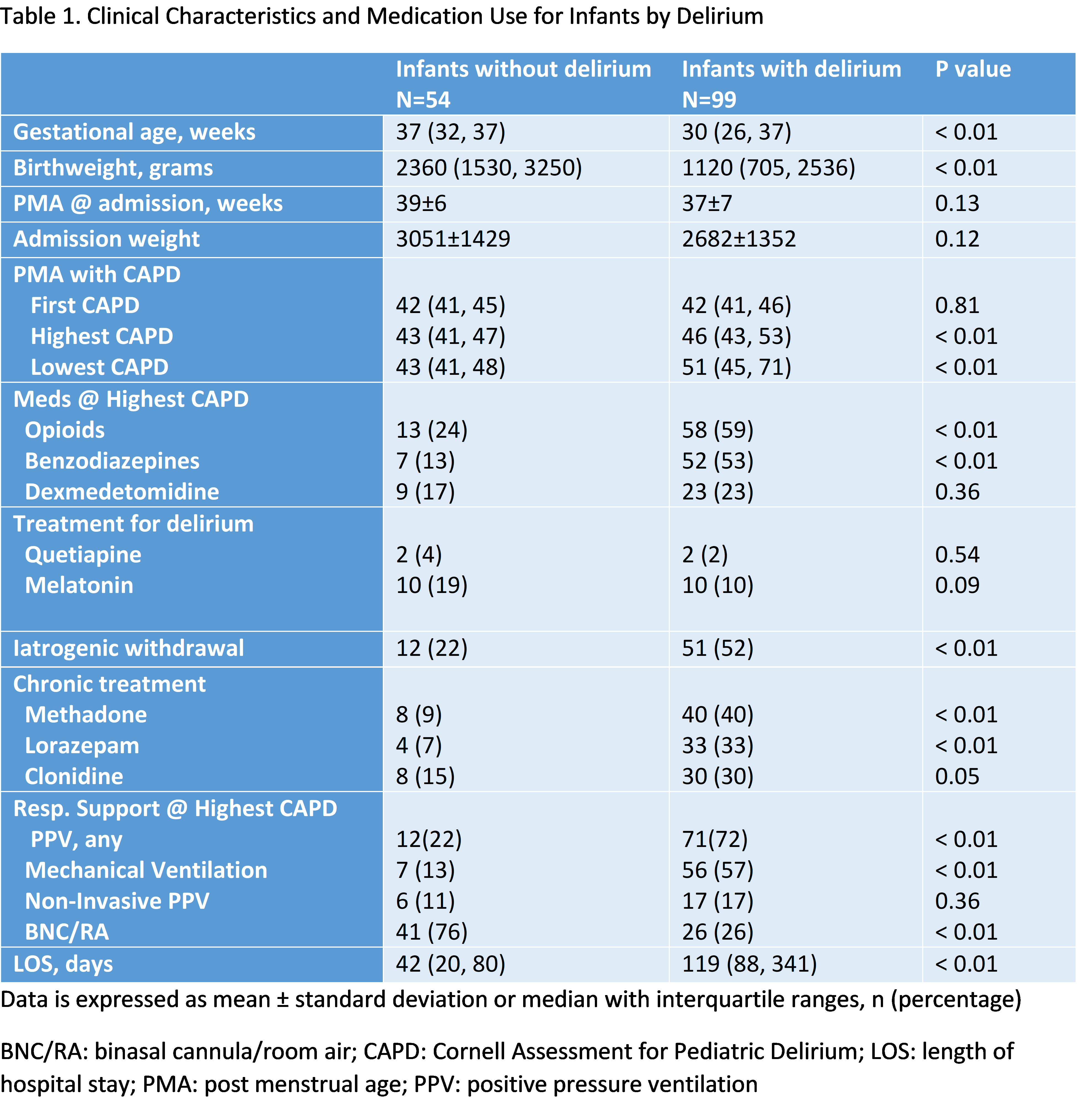
Figure 1: Delirium and Benzodiazepine with Gestational Age at Birth
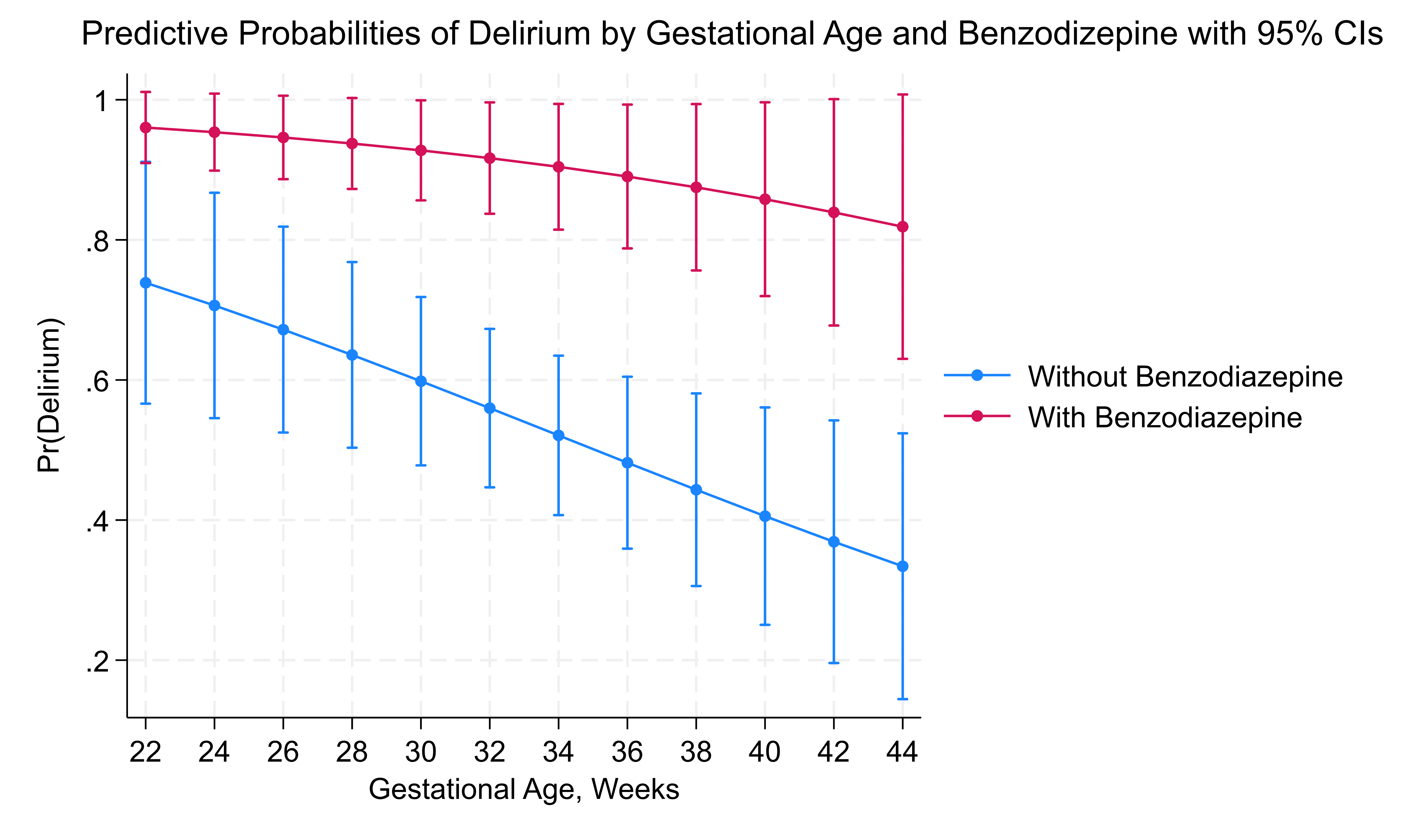
Figure 2: Delirium and Benzodiazepine with LOS