Immunizations/Delivery 1
Session: Immunizations/Delivery 1
675 - Predictors of Nirsevimab Uptake Across a Large Pediatric Primary Care Network
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 675.6450
Mahaa M. Ahmed, Children's Hospital of Philadelphia, Cherry Hill, NJ, United States; Torsten Joerger, Children's Hospital of Philadelphia, Philadelphia, PA, United States; Ziyi Wang, Children's Hospital of Philadelphia, Philadelphia, PA, United States; yun li, University of Pennsylvania, wynnewood, PA, United States; Jeremy Michel, Childrens Hospital of Philadelphia, Bryn Mawr, PA, United States; Jeffrey Gerber, Childrens Hospital of Philadelphia, Philadelphia, PA, United States

Ziyi Wang, MS
Statistician
Children's Hospital of Philadelphia
Exton, Pennsylvania, United States
Presenting Author(s)
Background: Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infections and hospitalizations among infants. Nirsevimab is a monoclonal antibody to the RSV fusion protein that received U.S. FDA approval in July 2023 and recommended for all children less than 8 months old. Given limited supply of nirsevimab during the 2023-2024 RSV season, it is important to examine potential inequities in its distribution.
Objective: To examine the distribution of nirsevimab during the 2023-2024 RSV season (October 1, 2023 to March 31, 2024) across the Children’s Hospital of Philadelphia (CHOP) Primary Care Network, including 32 practices with over 250 clinicians serving over 300,000 patients, and to develop and validate a predictive model to determine factors influencing nirsevimab receipt.
Design/Methods: Data were accessed from the CHOP electronic data warehouse; 100 random charts were reviewed to validate clinical and demographic variables of interest. Nirsevimab was available at all 32 practices starting October 16, 2023 without significant supply limitations for the season. To establish a birth cohort of infants eligible for nirsevimab who remined in the network, we included patients < 8 months old on October 1, 2023 with at least one primary care visit within 14 days of birth and at least one primary care visit after turning 8 months old or after the end of RSV season. Thus, (1) all of these children were eligible for nirsevimab and (2) none were born after nirsevimab was provided in the birth hospital (i.e. after October 1). Patient-level data were analyzed to develop a predictive model for receipt of nirsevimab using logistic regression.
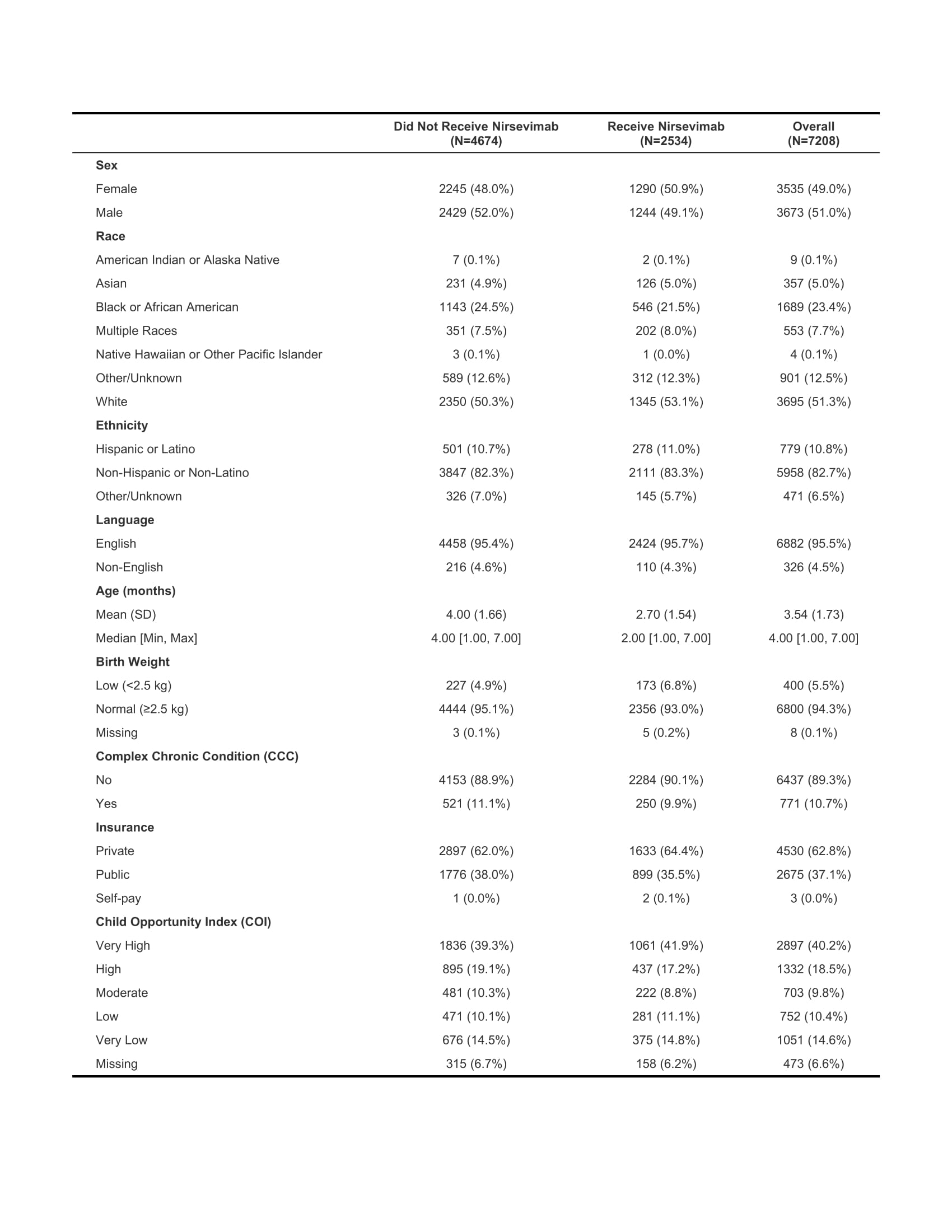
Results: Of 7208 eligible patients, 2534 (35%) received nirsevimab, ranging from 20%-65% across practices. Full cohort characteristics provided in Table 1. After adjustment, receipt of nirsevimab was associated with age at start of RSV season, race, and practice location (Figures 2 and 3). For each additional month of age, children were 39% less likely to receive nirsevimab (OR: 0.61, CI: 0.59, 0.63). Black patients were 46% less likely to receive nirsevimab compared to white patients (OR: 0.54, CI: 0.48, 0.66).
Conclusion(s): Despite adequate supply of nirsevimab, only 35% of eligible children received RSV prophylaxis. Use differed by practice location, patient age, and race. Future interventions should address these inequities to increase nirsevimab uptake for all.
Table 1. Cohort Characteristics
 All children across 32 CHOP primary care practices eligible for nirsevimab in the 2023-2024 RSV season
All children across 32 CHOP primary care practices eligible for nirsevimab in the 2023-2024 RSV seasonFigure 1. Unadjusted Nirsevimab Use by Practice
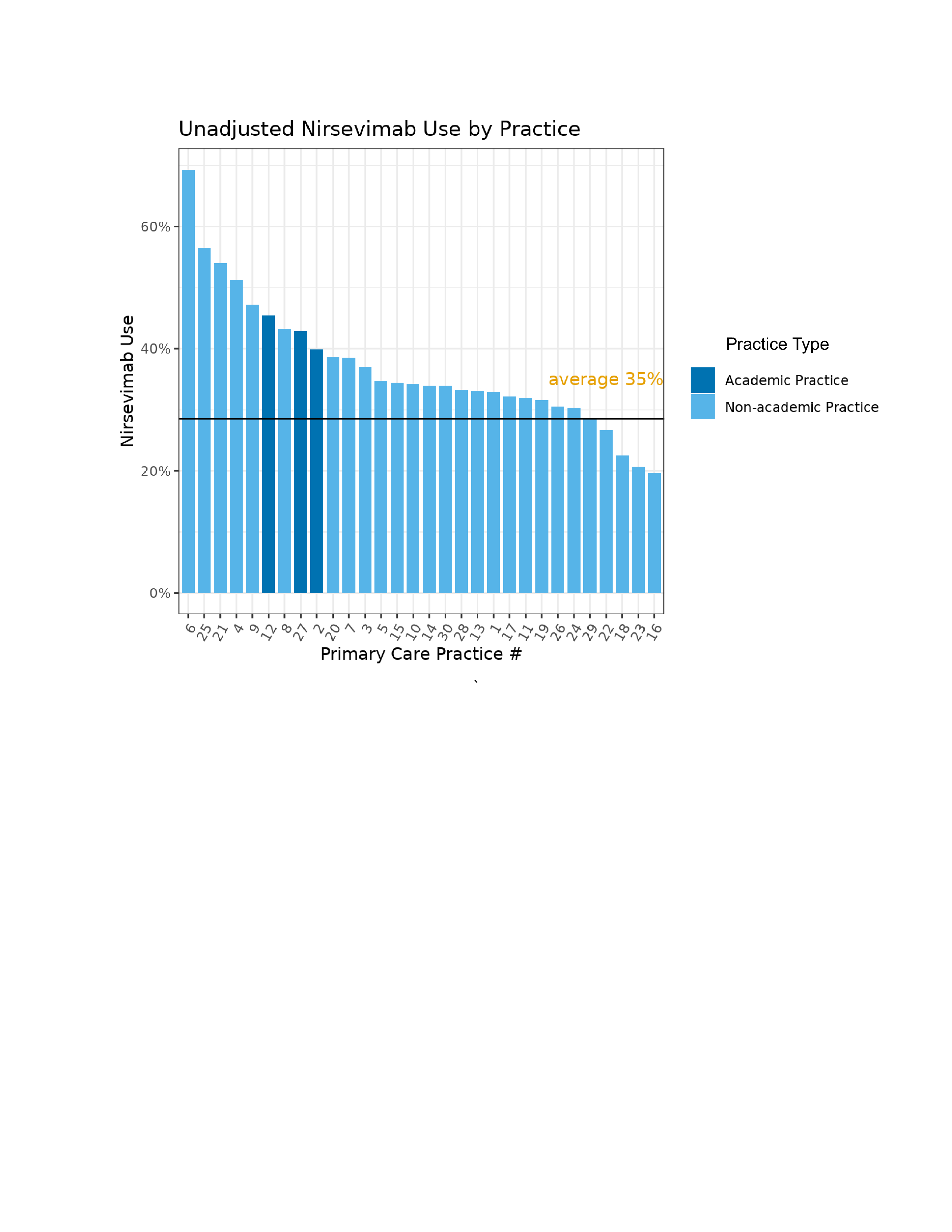 Practice #18 includes three different practices as they share the same group of clinicians
Practice #18 includes three different practices as they share the same group of cliniciansFigure 2. Adjusted Nirsevimab Use by Practice & Patient Characteristics.
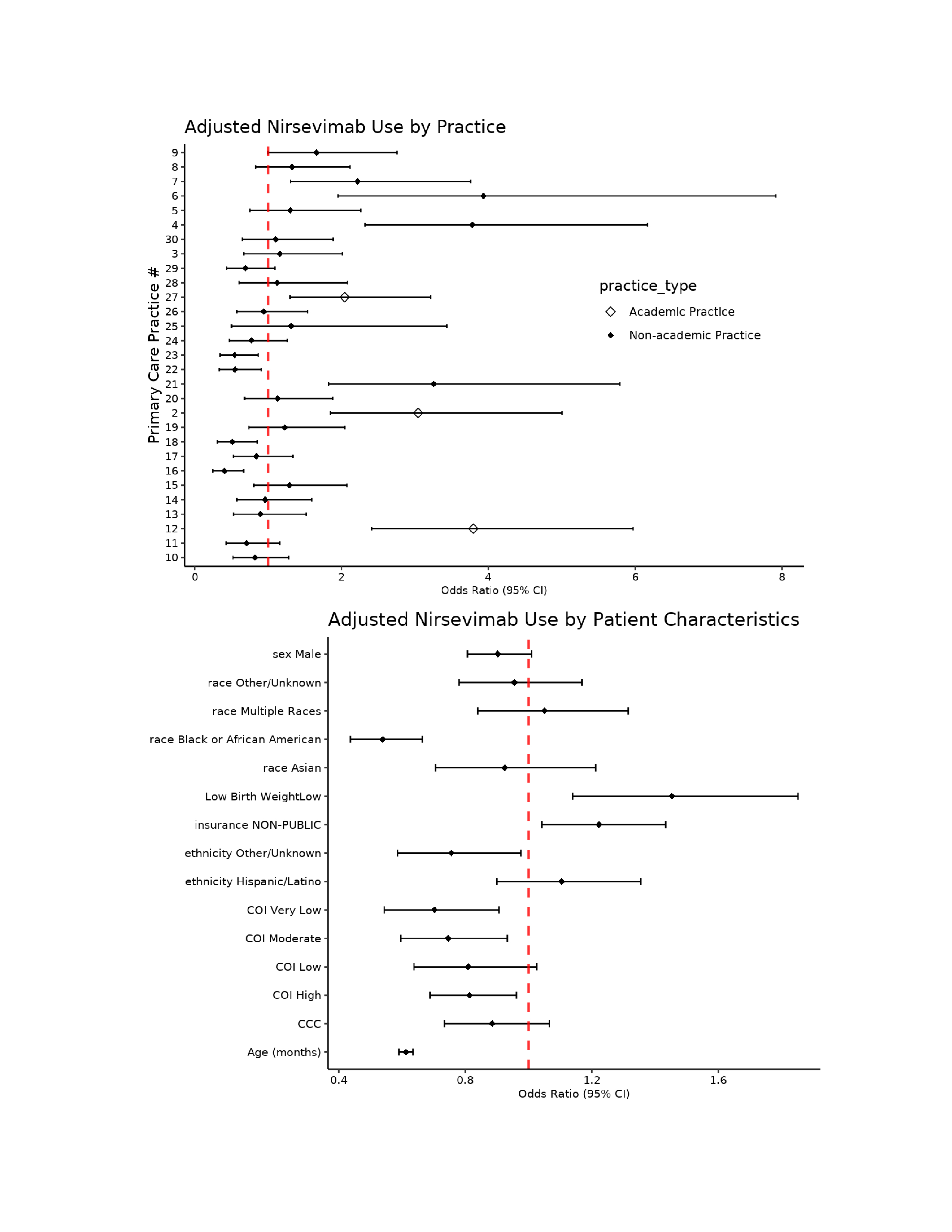 Top: Reference group: practice #1
Top: Reference group: practice #1Bottom: Reference group for sex: female; Reference group for race: white; Reference group for birth weight: normal; Reference group for insurance: public; Reference group for ethnicity: non-Hispanic/Latino; Reference group for COI: Very High

