Neonatal Neurology 2
Session: Neonatal Neurology 2
346 - Prediction of Language Scores at 2 Years Corrected Age While Still in the NICU: Construction and Evaluation of a Predictive Multimodal MRI Model at Term Equivalent Age for Infants Born Very Preterm
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 346.6236
Maria E. Barnes-Davis, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Katsuaki Kojima, Cincinnati Children's Hospital, Montgomery, OH, United States; Armin Allahverdy, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Abiot Y. Derbie, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Hailong Li, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Mekibib Altaye, Cincinnati Children's Hospital Medical Center, CINCINNATI, OH, United States; Lili He, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Nehal A. Parikh, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States

Maria E. Barnes-Davis, MD/PhD (she/her/hers)
Associate Professor and Attending Neonatologist
Cincinnati Children's Hospital Medical Center
Cincinnati, Ohio, United States
Presenting Author(s)
Background: Infants born very preterm ( < 32 weeks, VPT) are at risk for language disorder. Language is important to families, impacts many aspects of daily life, and has been hard to predict. Here, we develop and evaluate a model using clinical and multimodal MRI variables at term-equivalent age (TEA) for prediction of language scores at 2 years corrected age (CA) in a prospective regional cohort of infants born VPT.
Objective: To enable early and accurate prediction of language scores at 2 years CA from clinical data, conventional MRI, and multimodal quantitative MRI.
Design/Methods: Inclusion criteria included VPT birth. Exclusion criteria included cyanotic heart disease or congenital anomalies impacting the brain. We obtained non-sedated MRI on a single 3T scanner at 39-44 weeks postmenstrual age in 358 VPT. Our primary outcome was Language Composite from Bayley Scales of Infant & Toddler Development (BSID3) at 22-26 months CA. For each infant, we obtained resting state MRI functional connectivity (FC), diffusion MRI structural connectivity (SC), structural morphometry, global brain abnormality score (GBAS, Kidokoro 2013) and clinical data (Table 1). Nonnegative matrix factorization (NMF) was used to reduce the large number of variables to meaningful components. Specifically, NMF produced 30 SC, 28 FC, and 26 morphometry components, in addition to GBAS and 8 clinical variables. These 93 inputs per infant were analyzed with kernel-based support vector machine (kSVM) to predict language. We created 500 bootstrap datasets to evaluate model performance and correct for optimism (per Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis guidelines) assessing fit with adjusted R2 and root mean square error (RMSE, a measure of error between observed and expected).
Results: Complete data were available for 307 VPT (86%). Mean BSID3 Language Composite was 90.6. The model with only clinical data and GBAS accounted for 23% of variance (RMSE=17.27, Table 2). All models combining clinical data and advanced MRI had better RMSE and adjusted R2 (0.55-0.63). The model with the lowest RMSE (11.08) included clinical data, GBAS, SC, FC, and morphometry, explaining 55% of variance. Top MRI components included key areas for language development: bilateral temporal and parietal, cingulate, occipital, right fusiform, insula, and frontal areas.
Conclusion(s): Neonatal clinical variables, brain abnormality score, and quantitative MRI at TEA better predict language scores at 2 years for infants born VPT, demonstrating the predictive power of MRI at TEA for language using clinically available sequences.
Table 1: Demographic and Clinical Data for Included Participants
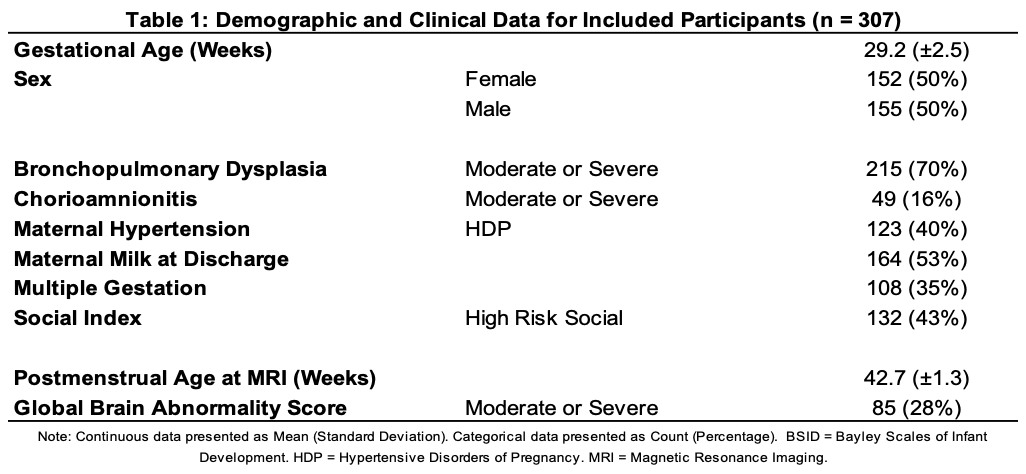
Table 2: Root Mean Square Error (RMSE) and Prediction Accuracy (Optimism-Corrected Adjusted R2) of Each Modality, Independently and in Combination
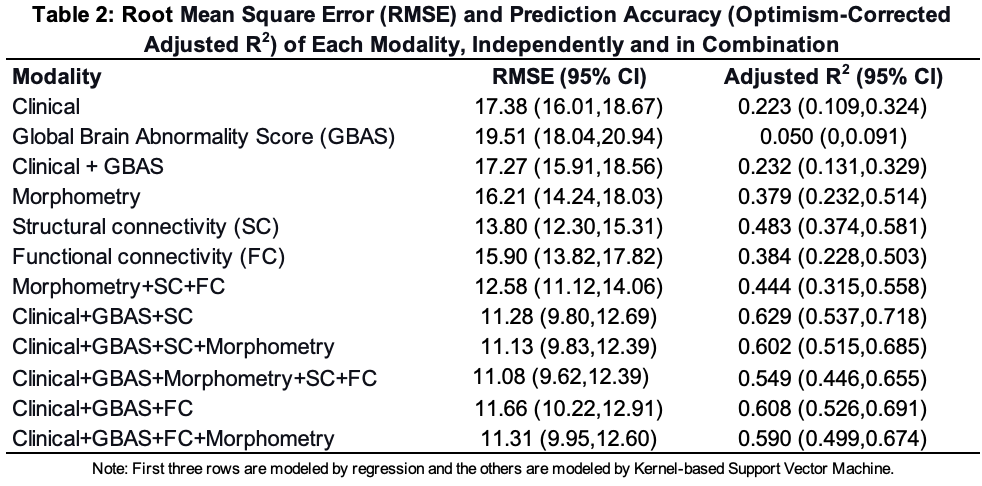
Figure 1: Top Inputs in kSVM Predictive Models and Visualization of Top Structural Connectivity Component Derived from Diffusion Magnetic Resonance Imaging Using NMF
.png) A. Top 10 inputs across modalities for the kernel-based support vector machine (kSVM) are ranked by partial R squared (a measure of the variance accounted for by that input). High Risk Social Score indicates that a given participant scored as high risk on a social index comprised of family structure, education and employment of primary caregiver, household income, primary language, and maternal age. Maternal Milk at Discharge indicates that the infant received any maternal milk at time of discharge from the hospital, regardless of amount. GBAS is the Global Brain Abnormality Score published by Kidokoro et al, 2013. Moderate/Severe Chorioamnionitis was determined based on placental pathology. Morphometry Component 15 included the left temporal lobe, left superior temporal gyrus (STG), right fusiform, right posterior cingulate, right temporal lobe, right medial and inferior temporal gyri, bilateral occipital lobes, left fusiform, left insula, and the brainstem. Structural Connectivity Component 5 included the left parietal lobe, bilateral cingulate, right parietal lobe, bilateral medial and inferior temporal gyri, right fusiform, left STG, bilateral frontal lobe white matter, right parahippocampal gyrus, right STG, right insula, and the left fusiform. Morphometry Component 24 included the left fusiform, the left medial and inferior temporal gyri, the brainstem, the bilateral temporal lobes, the bilateral STG, and the hippocampi and amygdala.
A. Top 10 inputs across modalities for the kernel-based support vector machine (kSVM) are ranked by partial R squared (a measure of the variance accounted for by that input). High Risk Social Score indicates that a given participant scored as high risk on a social index comprised of family structure, education and employment of primary caregiver, household income, primary language, and maternal age. Maternal Milk at Discharge indicates that the infant received any maternal milk at time of discharge from the hospital, regardless of amount. GBAS is the Global Brain Abnormality Score published by Kidokoro et al, 2013. Moderate/Severe Chorioamnionitis was determined based on placental pathology. Morphometry Component 15 included the left temporal lobe, left superior temporal gyrus (STG), right fusiform, right posterior cingulate, right temporal lobe, right medial and inferior temporal gyri, bilateral occipital lobes, left fusiform, left insula, and the brainstem. Structural Connectivity Component 5 included the left parietal lobe, bilateral cingulate, right parietal lobe, bilateral medial and inferior temporal gyri, right fusiform, left STG, bilateral frontal lobe white matter, right parahippocampal gyrus, right STG, right insula, and the left fusiform. Morphometry Component 24 included the left fusiform, the left medial and inferior temporal gyri, the brainstem, the bilateral temporal lobes, the bilateral STG, and the hippocampi and amygdala. B. Regions included in Structural Connectivity Component 5 generated by unsupervised nonnegative matrix factorization (NMF) are highlighted here on a 40 week postmenstrual age template brain from the Developing Human Connectome Project (dHCP). The structural connectivity (SC) components are based on structural connectivity analyses between the 86 regions in the dHCP atlas derived from fractional anisotropy (FA) values from 36 direction diffusion magnetic resonance imaging (dMRI) acquisitions at term equivalent age. Here, we show the regions that are included in SC Component 5 (the highest ranked SC input) with 7 axial slices marked on the corresponding 3D brain.

