Neonatal General 1: Respiratory, BPD
Session: Neonatal General 1: Respiratory, BPD
267 - Sedative Use in Infants with Grade 3 Bronchopulmonary Dysplasia (BPD) and Associations with Neurodevelopmental Therapies
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 267.6392
Thomas Davis, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Divya Rana, UTHSC, Memphis, TN, United States; Audrey Miller, Nationwide childrens hospital, Columbus, OH, United States; Meaghan Ransom, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States; Bridget DiPrisco, Keck School of Medicine of the University of Southern California, Santa Monica, CA, United States; Karishma Rao, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Tara L. DuPont, University of Utah, Salt Lake City, UT, United States; Melissa Hanin, Nationwide Children's Hospital, Bexley, OH, United States; Melissa House, Emory University School of Medicine, Atlanta, GA, United States; Brianna K.. Brei, University of Nebraska College of Medicine, Omaha, NE, United States; Susan Gage, CHOC Children's Hospital of Orange County, Orange, CA, United States; Matthew Coyle, Childrens Hospital of Philadelphia, Springfield, PA, United States; Sara B.. DeMauro, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States

Thomas Davis, MD (he/him/his)
Pediatric Resident
Childrens Hospital of Philadelphia
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: Infants born preterm with BPD experience prolonged hospitalizations, often with exposure to multiple sedating medications. Sedative medications may increase risk of neurodevelopmental impairment (NDI), either directly or by impacting participation in developmental therapies during a critical window of development. However, few studies detail their contemporary use or associations between sedation exposure and therapies to support neurodevelopment in hospitalized infants with grade 3 BPD.
Objective: To describe patterns of sedation exposure by level of respiratory support and relationships between exposure and neurodevelopmental therapies (PT/OT/Speech) in infants with grade 3 BPD.
Design/Methods: We performed a point prevalence survey at 22 NICUs that participate in the BPD Collaborative. Eligible infants were >36 weeks postmenstrual age (PMA), intubated at 36 weeks PMA (grade 3 BPD), < 24 months corrected age, and never discharged. Infants with culture positive sepsis, tracheostomy, or had major surgery in 7 days prior were excluded. Data were collected from chart review or bedside evaluation and analyzed using descriptive statistics.
Results: Of 141 infants, 97 (69%) had received one sedating medication in the prior 48hrs; 73 (75%) of these received more than one (mean 2.8+/-1.6) (Table 1). The most common agents were ⍺-agonists (n=67, 48%) and benzodiazepines (n=65, 46%) with the combination of ⍺-agonist, opioid, and benzodiazepine the most common treatment overall (n=27, 19%) (Fig 1). Any exposure and number of medications in past 48hrs among those exposed were both significantly associated with level of respiratory support (p < 0.001). There was an association between number of therapy sessions completed in prior 48 hours and exposure to any sedating medications (Wilcoxon Rank Sum z=3.3, p< 0.001)
Conclusion(s): The majority of hospitalized infants with grade 3 BPD are exposed to sedative medications, and polypharmacy is common. Higher levels of respiratory support were associated with increased sedation exposure, though further evaluation is needed to determine whether tracheostomy leads to less exposure to sedation compared to those with ETT. Sedation exposure was associated with fewer completed therapy sessions. Future work aims to unravel the impact of respiratory support and sedation exposure and understand the consequences of deferral of early neurodevelopmental therapies in infants with grade 3 BPD.
Table 1. Demographics
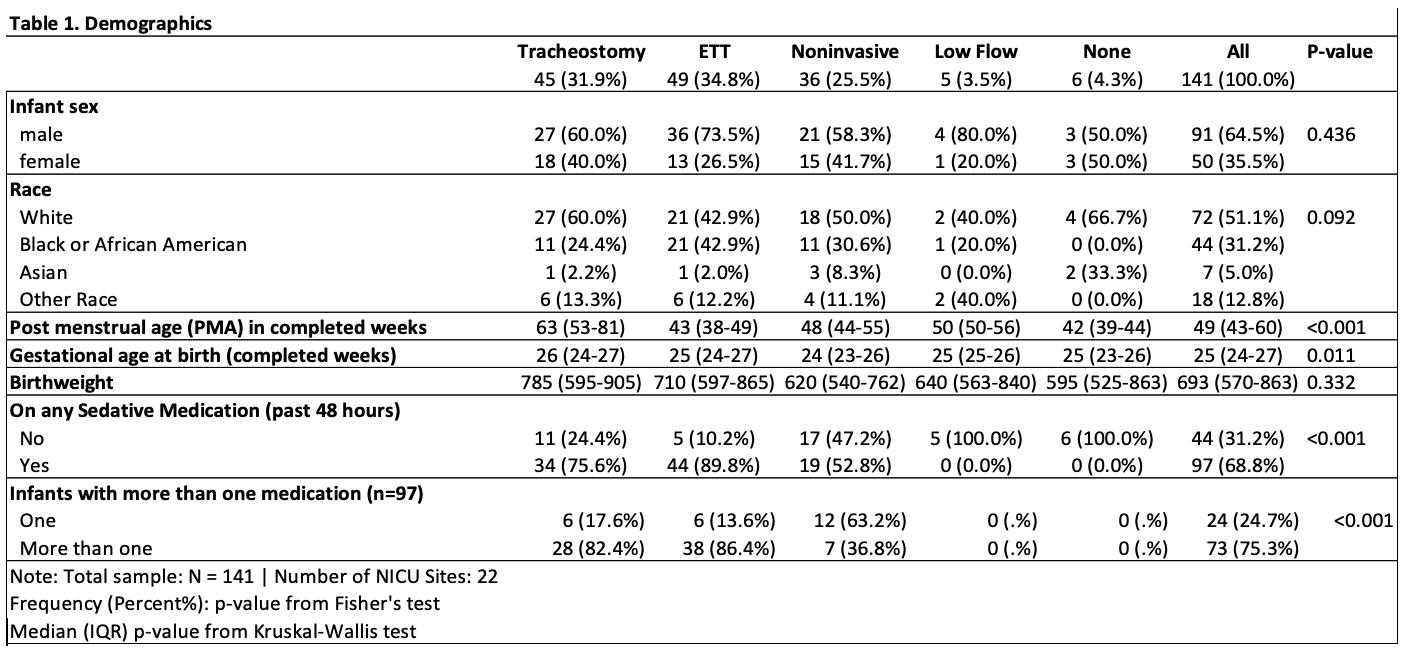
Figure 1: Numbers of Infants with Grade 3 BPD Treated with Sedating Medications
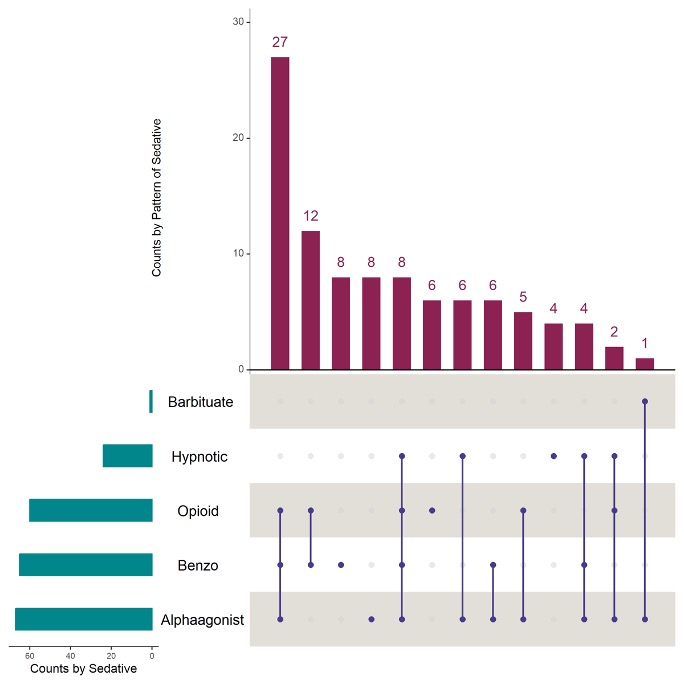 The horizontal green bars at the bottom of the graph represent the number of patients receiving medications from each drug class. The vertical red bars represent the numbers of infants receiving each combination of medications, with blue bars below depicting each combination of medication exposures.
The horizontal green bars at the bottom of the graph represent the number of patients receiving medications from each drug class. The vertical red bars represent the numbers of infants receiving each combination of medications, with blue bars below depicting each combination of medication exposures.
