Neonatal General 3: NICU Practices
Session: Neonatal General 3: NICU Practices
546 - Line Insertion Through a Long Umbilical Stump, a Concept Generating Laboratory Study
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 546.5728
Itamar Nitzan, Shaare Zedek Medical Center, Jerusalem, Yerushalayim, Israel; Yshia (Shaya) Langer, Hadassha University Medical Center, Jerusalem, Ephrat, Yerushalayim, Israel; יאיר קסירר, מגדל עדר 8, NEVE DANIEL, Yerushalayim, Israel; Lea Liat L. Nasich, Shaare Zedek Medical Center, Jerusalem, Yerushalayim, Israel; Robert I. Koppel, Cohen Children's Medical Center, New Hyde Park, NY, United States; Francis B. Mimouni, Tel aviv University, Tel Aviv, Tel Aviv, Israel; Anup Katheria, Sharp Mary Birch Hospital for Women & Newborns, San Diego, CA, United States
- IN
Itamar Nitzan, MD (he/him/his)
neonatologist
Shaare Zedek Medical Center
Jerusalem, Yerushalayim, Israel
Presenting Author(s)
Background:
Background: Preterm infants require early Intravenous (IV) fluid. This typically requires percutaneous insertion of an IV cannula which is technically challenging and involves pain for the baby. For umbilical line insertion, the abdomen of the baby is commonly disinfected with agents that may cause skin irritation and burns and involves discomfort that is considered harmful for preterm infants. We hypothesized that a 10-20 cm umbilical cord segment can be catheterized with an appropriate guide wire (figure 1). This may facilitate line establishment with minimal (if any) baby discomfort. In addition, we hypothesized that a Venflon catheter can be inserted into the UV to enable rapid fluid administration.
Objective: To assess whether a long segment of the umbilical cord can be catheterized.
Design/Methods: A laboratory proof of concept study, performed on umbilical cord segments removed from babies after delivery. In phase 1, we used variety of techniques to determine an ideal method for long segment umbilical vein catheterization. In phase 2, we documented rate of successful catheter advancement using this method.
In phase 2, the cannulation procedure was as follows:
1. The UV was cannulated with a Venflon
2. A guidewire micropuncture introducer set 0.18"/40cm or the 60 CM Cope Mandril wire guide (Cook Medical Bloomington Indiana, USA) was introduced and advanced until resistance was felt.
3. The insertion point was dilated with a dilator.
4. The dilator was removed, and the line was advanced upon the guide wire.
5. If the guidewire was stuck at a point of resistance less than 20 cm from cannulation point, further attempts to advance the guidewire after inserting the line were performed (Fig. 2).
Results: We succeeded advancing the catheter from the insertion point to the cord proximal end, an average distance of 15.7±3.5cm, in 7 out of 10 cord segments.
Venflon insertion into the UV was successful in all 10 cords. The Venflon can be easily taped and secured to cord clamp applied on the cord distal to the Venflon insertion point.
Conclusion(s): It is possible to advance a catheter within the umbilical vein through an umbilical cord long segment. Larger laboratory studies are needed to determine parameters, such as the degree of UV coiling, that may influence success or failure of the method. Clinical studies are required to evaluate whether this method may facilitate minimal handling insertion of umbilical venous catheters.
Figure 1
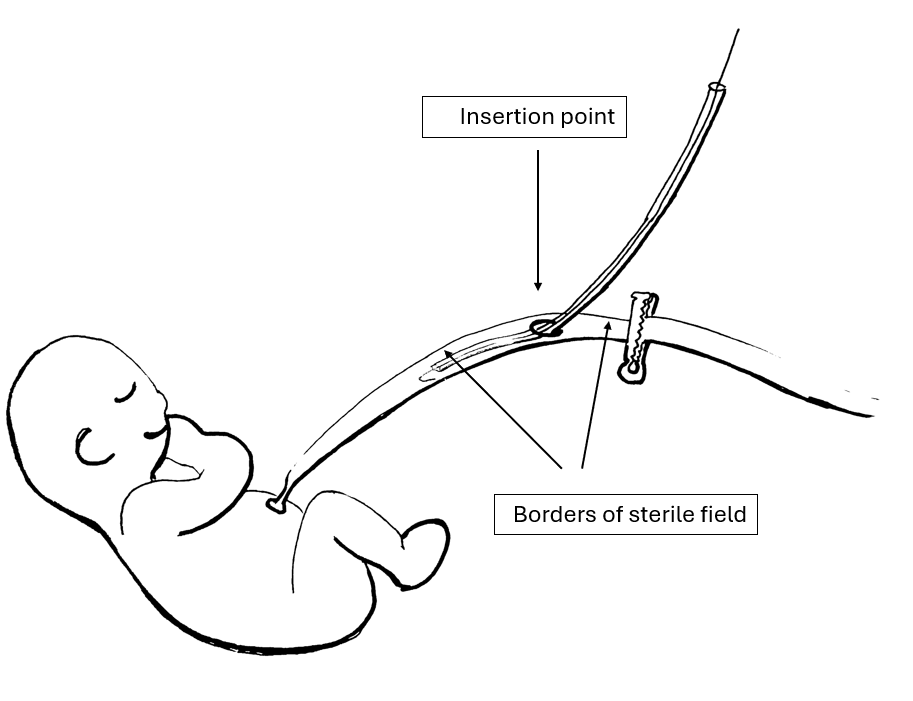 Schematic drawing of the planned method for line insertion.
Schematic drawing of the planned method for line insertion.Figure 2
.png) Canulation of a removed umbilical cord during the study.
Canulation of a removed umbilical cord during the study.
