Neonatal General 2: Neurology
Session: Neonatal General 2: Neurology
315 - Optimizing Dexmedetomidine Dosing for Sedation in Neonates Undergoing Therapeutic Hypothermia
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 315.5652
Terrence CK. Lo, The Hospital for Sick Children, Richmond Hill, ON, Canada; Han Byul Kang, The Hospital for Sick Children, Toronto, ON, Canada; Carol McNair, The Hospital for Sick Children, Toronto, ON, Canada; Diane Wilson, Hospital for Sick Children, Toronto, ON, Canada; Jennifer Bizzarri, The Hospital for Sick Children, Toronto, ON, Canada; Brian T. Kalish, Boston Children's Hospital, Boston, MA, United States; Emily WY. Tam, The Hospital for Sick Children, Toronto, ON, Canada; Winnie TW. Seto, Hospital for Sick Children, Toronto, ON, Canada; Erin Chung, The Hospital for Sick Children, Toronto, ON, Canada

Terrence Lo, BMSc, PharmD, ACPR, RPh (he/him/his)
Pharmacist
The Hospital for Sick Children
Richmond Hill, Ontario, Canada
Presenting Author(s)
Background: Hypoxic-ischemic encephalopathy (HIE) is one of the leading causes of neonatal death and is treated with therapeutic hypothermia (TH). Opioids are often prescribed for sedation and to suppress shivering but are associated with significant adverse effects including neurodevelopmental impairment. Recently, opioids have been replaced by dexmedetomidine due to favourable effectiveness and safety outcomes in neonatal intensive care unit (NICU) patients. However, the optimal dose of dexmedetomidine remains unclear.
Objective: To describe dosing patterns, evaluate compliance, and compare effectiveness and safety between the revised in-house dosing guideline (intervention) of dexmedetomidine 0.2µg/kg/h with titration by 0.1µg/kg/h every 30-60 minutes as needed and the original in-house dosing guideline (comparator) of dexmedetomidine 0.05µg/kg/h with titration by 0.05µg/kg/h every six hours as needed.
Design/Methods: This was a retrospective cohort study that included neonates with HIE admitted to a level IV NICU for TH who received dexmedetomidine. Dexmedetomidine doses, effectiveness endpoints (opioid/benzodiazepine exposures; Neonatal Pain, Agitation, and Sedation Scale [N-PASS] scores; and shivering), and safety endpoints (hypotension and bradycardia) were analyzed.
Results: A total of 106 neonates (n=56 in intervention and n=50 in comparator groups) were included. Compliance to intervention was 69.6% for initial dexmedetomidine doses, 62.5% for dose increases, and 44.6% for dose decreases. An increased odds of opioid exposure was observed in the intervention group, but with a wide confidence interval (CI) (adjusted odds ratio: 7.13, 95% CI: 1.59-51.2, p=0.02, vs. comparator). A greater proportion of patients in the intervention group had bradycardia (21.4% vs. 4%, p=0.03). No significant differences in opioid/benzodiazepine exposures, shivering episodes, suboptimal N-PASS scores, or hypotension events were observed (p≥0.05).
Conclusion(s): The revised dexmedetomidine dosing guideline was associated with similar effectiveness outcomes, but more bradycardia compared with the original dosing. A prospective study with population pharmacokinetic analysis is needed to individualize dexmedetomidine dosing for neonates with HIE undergoing TH.
Patient Selection
.jpg) DUE, drug use evaluation; HIE, hypoxic-ischemic encephalopathy; NICU, neonatal intensive care unit; OR, operating room; TH, therapeutic hypothermia
DUE, drug use evaluation; HIE, hypoxic-ischemic encephalopathy; NICU, neonatal intensive care unit; OR, operating room; TH, therapeutic hypothermiaCumulative MED During Dexmedetomidine Infusion
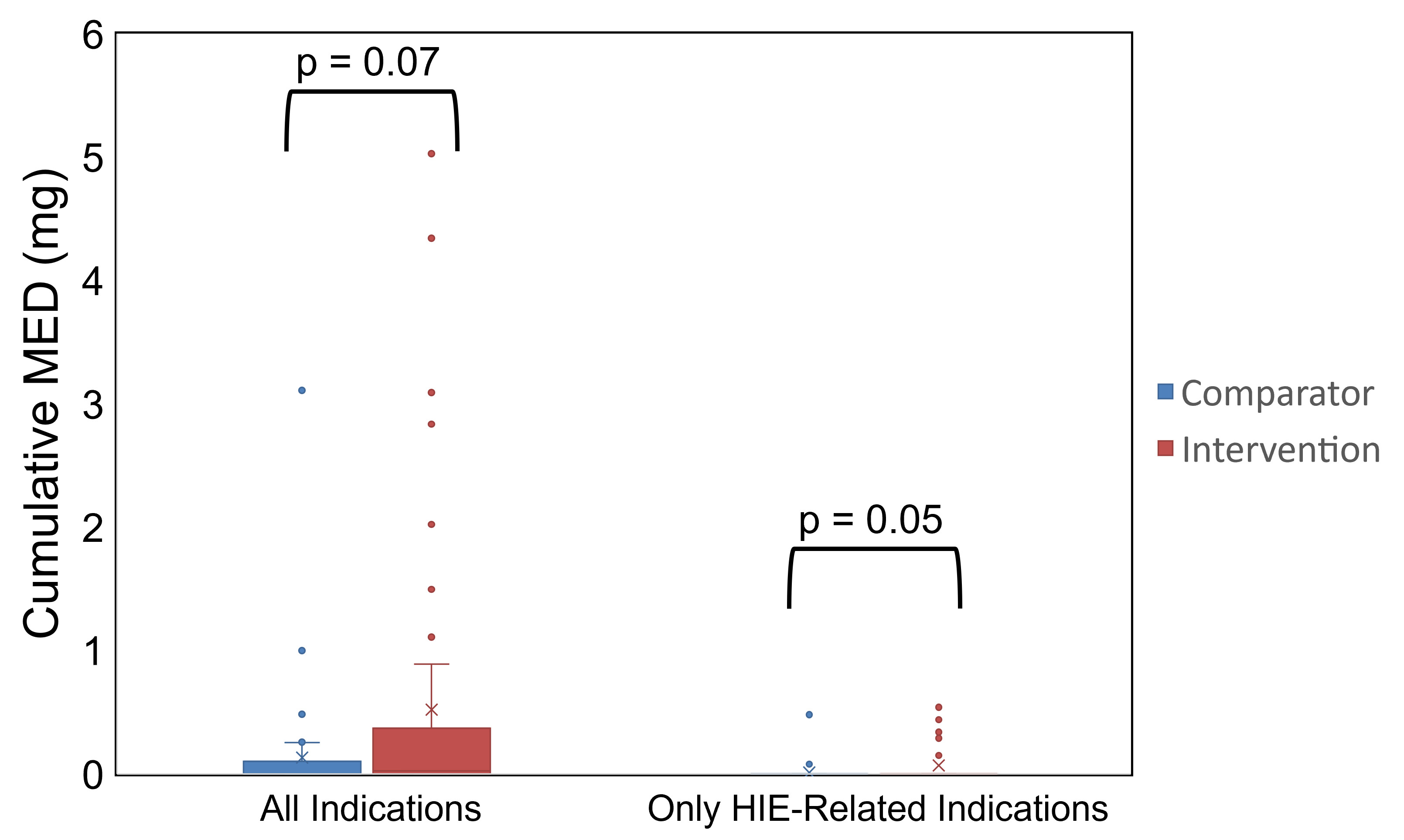 MED, morphine equivalent dose
MED, morphine equivalent doseCumulative Midazolam Equivalent Dose During Dexmedetomidine Infusion
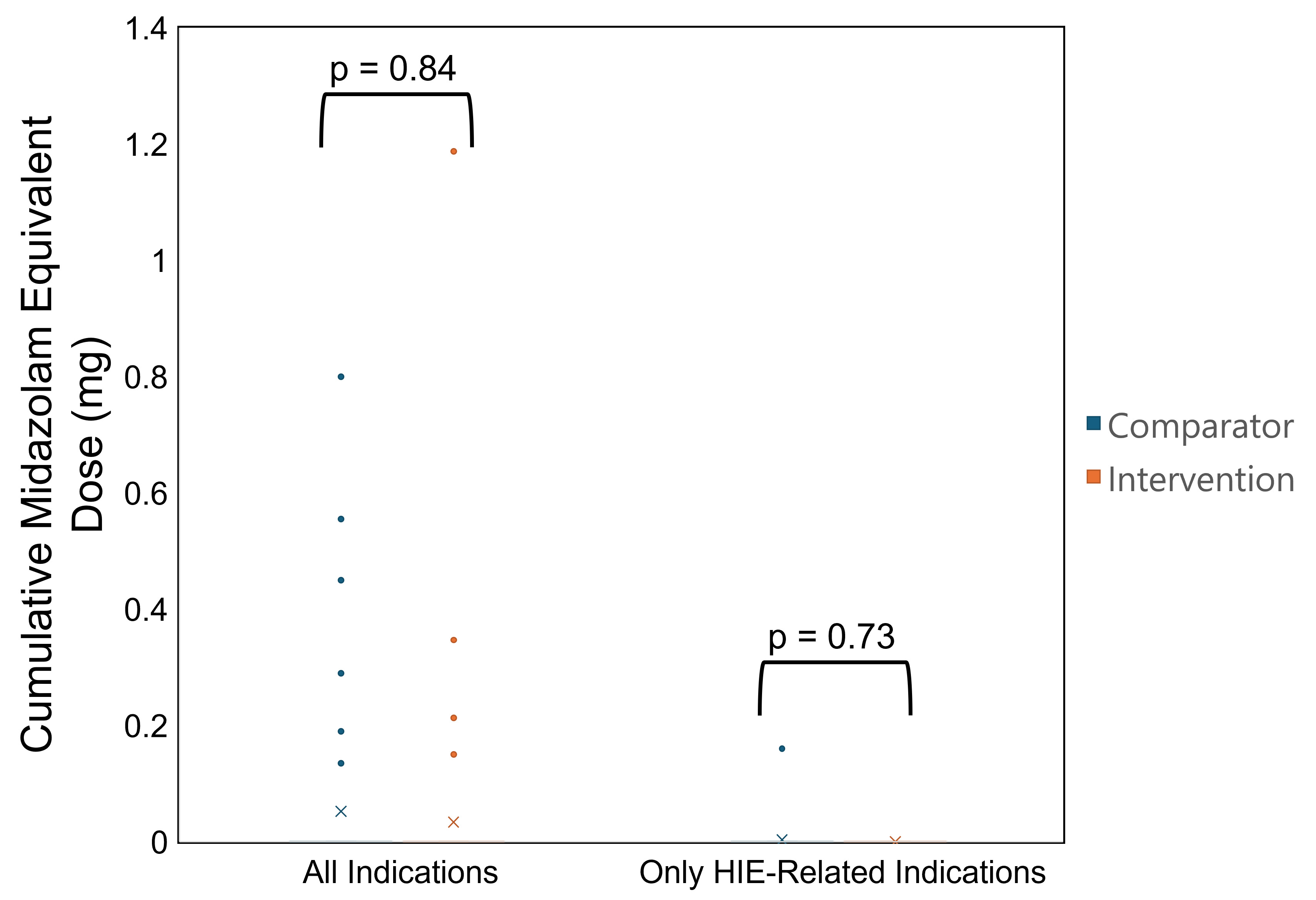
Patient Selection
.jpg) DUE, drug use evaluation; HIE, hypoxic-ischemic encephalopathy; NICU, neonatal intensive care unit; OR, operating room; TH, therapeutic hypothermia
DUE, drug use evaluation; HIE, hypoxic-ischemic encephalopathy; NICU, neonatal intensive care unit; OR, operating room; TH, therapeutic hypothermiaCumulative MED During Dexmedetomidine Infusion
 MED, morphine equivalent dose
MED, morphine equivalent doseCumulative Midazolam Equivalent Dose During Dexmedetomidine Infusion


