Neonatal Neurology 3
Session: Neonatal Neurology 3
367 - Efficacy and Safety of Dexmedetomidine as an Opioid Alternative for Sedation in Neonates with HIE Undergoing Therapeutic Hypothermia
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 367.4757
Athanasios Chamzas, Center for Translational Medicine, University of Maryland Baltimore, Baltimore, MD, United States; Fulden Aycan, University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States; Mathangi Gopalakrishnan, University of Maryland, Baltimore, MD, United States; Dina El-Metwally, University of Maryland School of Medicine, Baltimore, MD, United States

Dina El-Metwally, MD, PhD
Division Chief, Neonatology
University of Maryland School of Medicine
Baltimore, Maryland, United States
Presenting Author(s)
Background: Therapeutic hypothermia (TH) is the standard of care to reduce the risk of death or disability in hypoxic-ischemic encephalopathy (HIE) infants. Sedation during TH typically uses opioids like fentanyl or morphine, which may cause adverse effects. Dexmedetomidine (DEX), a selective alpha-2 adrenergic agonist, provides sedation and analgesia without affecting respiratory drive or gastrointestinal motility, making it a promising non-opioid alternative for neonatal sedation during TH.
Objective: To evaluate the efficacy and safety of dexmedetomidine for sedation in neonates with HIE undergoing TH compared to used opioid regimens using real-world data.
Design/Methods: A retrospective cohort study was conducted using electronic medical records of neonates with HIE undergoing TH at the University of Maryland Medical Center from 2018-2024. Patients were divided into two groups: those who received dexmedetomidine with opioids as needed and those who received only opioids (fentanyl/morphine) for sedation.
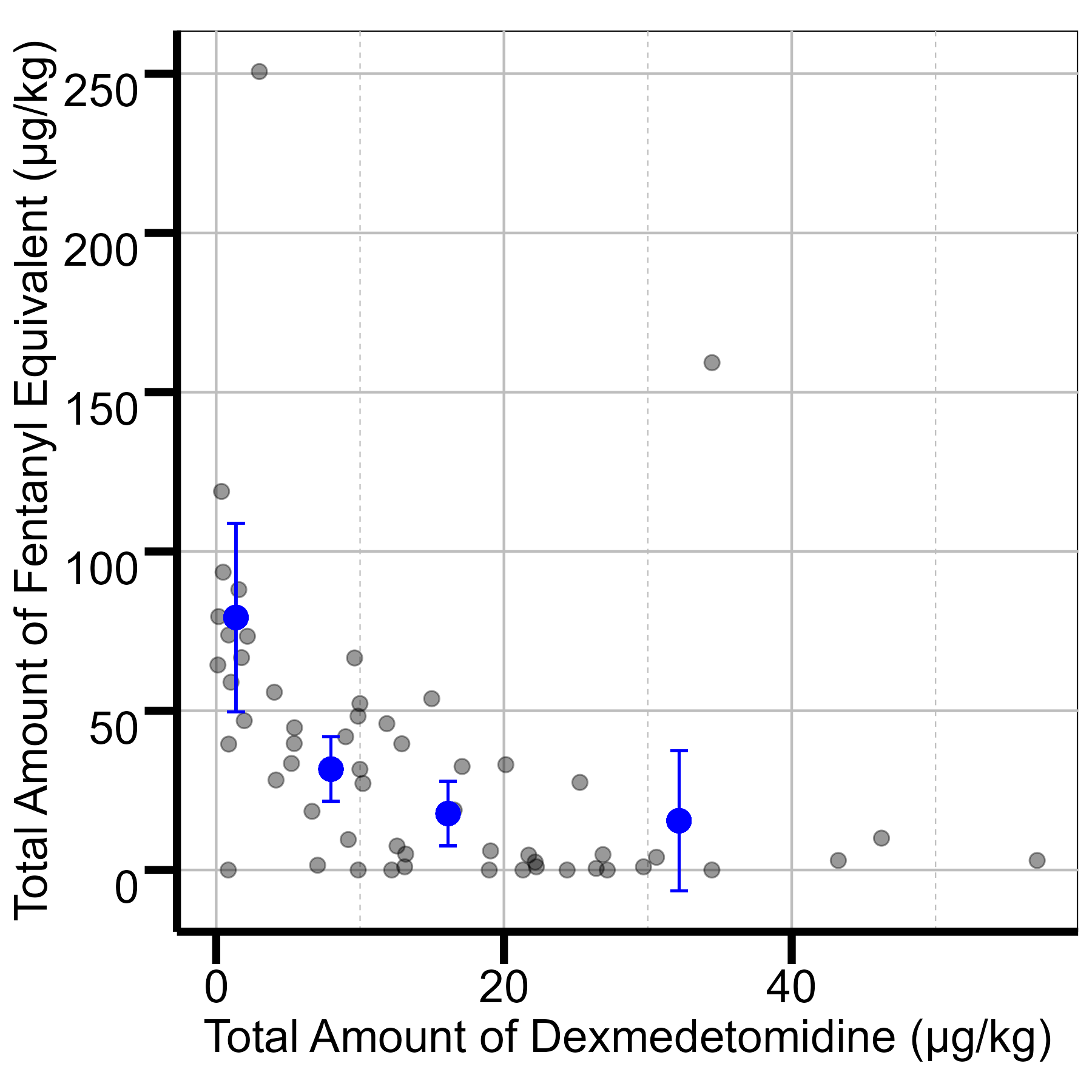
Results: A total of 159 neonates were included: 103 in the opioid-only group and 56 in the dexmedetomidine group, with similar baseline demographics. During hypothermia, the dexmedetomidine group had a 41% reduction in overall opioid exposure (71.3 vs. 42.0 μg/kg, p < 0.001). Time to first opioid bolus was increased (7.5 vs. 13.6 hours, p = 0.01), showing sustained sedation efficacy. The mean total amount and number of opioid bolus doses were reduced in the dexmedetomidine group (from 6.2 to 4.4 μg/kg [29% reduction], p=0.11 and from 4.3 to 3.1 [28% reduction], p=0.10, respectively), suggesting a decreased need for additional opioid administration. Sedation levels assessed by NPASS scores were comparable between dexmedetomidine and opioids (0.98 ± 0.86 vs. 1.19 ± 0.88, p = 0.16, respectively), indicating reduced opioid use did not compromise sedation quality. Regarding safety, no notable differences in hemodynamic parameters or adverse events were observed, except for a slight reduction in mean heart rate in the dexmedetomidine group during hypothermia (107 vs. 101 bpm, p < 0.01), though both remained within normal neonatal ranges (>100 bpm).
Conclusion(s): Dexmedetomidine is a safe and effective non-opioid alternative for sedation in neonates with HIE undergoing TH. It significantly reduced opioid exposure and delayed the need for additional sedation without compromising sedation efficacy or increasing adverse events. These findings support its use in sedation guidelines for this population to minimize opioid-related side effects.
Table 1: Baseline Demographics
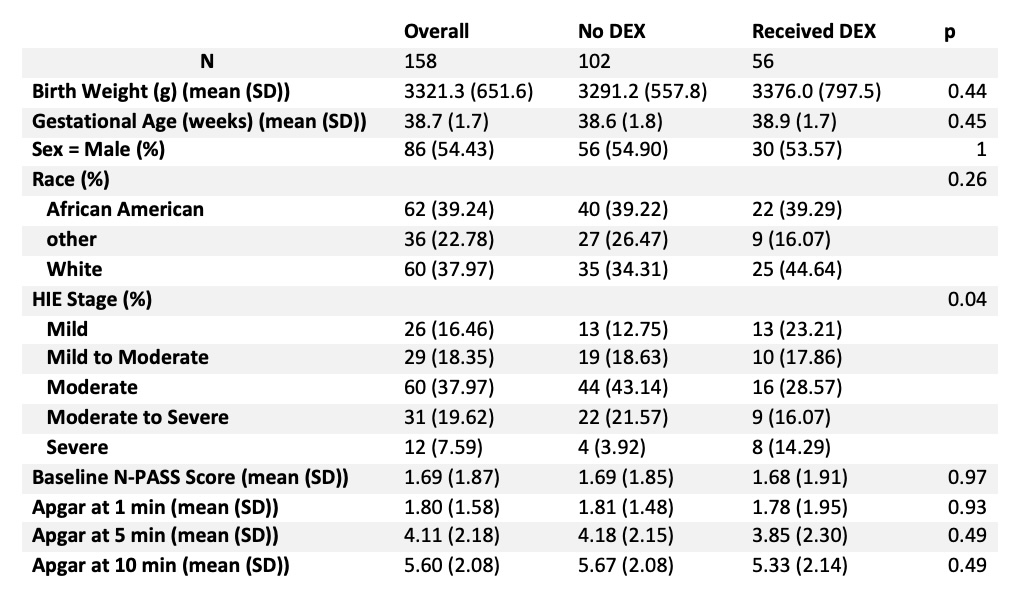 DEX: Dexmedetomidine
DEX: DexmedetomidineTable 2: Comparison between Dexmedetomidine group and Fentanyl Equivalent group
.jpg) DEX: Dexmedetomidine
DEX: DexmedetomidineFigure 1: Total amount of Dexmedetomidine vs Fentanyl Equivalent