Emergency Medicine 4
Session: Emergency Medicine 4
249 - Intravenous ketamine for emergency department treatment of suicidal ideation in a paediatric population: a double-blind, randomised, placebo-controlled, parallel-arm pilot trial (KSI study)
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 249.5196
Michael Schlegelmilch, University of Calgary, Calgary, AB, Canada; Amy Plint, University of Ottawa Faculty of Medicine, Ottawa, ON, Canada; Clare Gray, University of Ottawa Faculty of Medicine, Ottawa, ON, Canada; Nicholas Barrowman, University of Ottawa Faculty of Medicine, Ottawa, ON, Canada; Tyrus Crawford, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada; Stephen A. Kutcher, Childrens Hospital of Eastern Ontario, Montreal, PQ, Canada; Maala Bhatt, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada
- MS
Michael Schlegelmilch, MD, MPH (he/him/his)
Pediatric Emergency Medicine Physician/Clinical Lecturer
University of Calgary
Calgary, Alberta, Canada
Presenting Author(s)
Background: Suicidal ideation (SI) is a common reason for visits to the emergency department (ED) by adolescents. While intravenous (IV) ketamine rapidly reduces SI in adults, its efficacy in adolescents remains unstudied.
Objective: To assess the feasibility of a trial of a single dose IV ketamine to reduce adolescent SI in the ED.
Design/Methods: We conducted a double-blind, randomized, placebo-controlled pilot trial from Jan-May 2024 in a Canadian pediatric ED. Eligible participants were 12 to 17 years with moderate to severe SI (current thoughts of killing themselves, Beck Scale for SI (SS15) score >3/10) and were medically stable. Exclusion criteria were intellectual disability, active psychosis, contraindications to ketamine, or being under an involuntary psychiatric hold. Participants were randomized to IV ketamine (0.5 mg/kg; max 50 mg) or IV normal saline (0.5 ml/kg; max 50 mL), infused over 40 minutes. SI severity was measured at baseline (just prior to T-0, start of the infusion), at 40-, 80-, and 120-minutes after the infusion started (T-40, T-80, T-120) and on days 1 and 7 using three assessment tools: SSI5, Montgomery-Asberg Depression Rating Scale (MADRS10), and Beck Depression Inventory (BDI9). After T-120, participants received the usual care from the ED mental health team. Feasibility was evaluated by recruitment success and completion of the infusion/follow-up. The primary clinical outcome was SI severity at T-40. Additional outcomes were SI severity at all follow-up times, hospital admission at enrollment, ED revisits, and death within 30 days. Adverse events and dissociation were monitored. The planned sample size was 20 participants.
Results: Of 129 potentially eligible participants, 73 were screened, 20 were eligible, and all consented. All participants completed the infusion and follow up to day 1. 18 participants (90%) completed day 7 follow-up. There were no significant differences in SI severity between groups at T-40 (SSI5: P=0.06; MADRS10: P=0.19; BDI9: P=0.18) nor at other follow-up points (Table 1). Hospital admission at enrollment was 0/10 (0%) and 4/10 (40%) in the ketamine and control groups respectively. 30-day ED return visits were 7/10 (70%) for ketamine and 1/10 (10%) for control. No serious adverse events or deaths occurred. Dissociation was reported by 9/10 (90%) of the ketamine group and 4/10 (40%) of controls during the infusion; some reported dissociation prior to the start of the infusion (3/10 ketamine, 1/10 control).
Conclusion(s): Recruiting adolescents to an ED-based IV ketamine study for SI is feasible. A larger trial is needed to clarify potential clinical benefits.
Table 1. Mean difference between groups in suicidal ideation severity
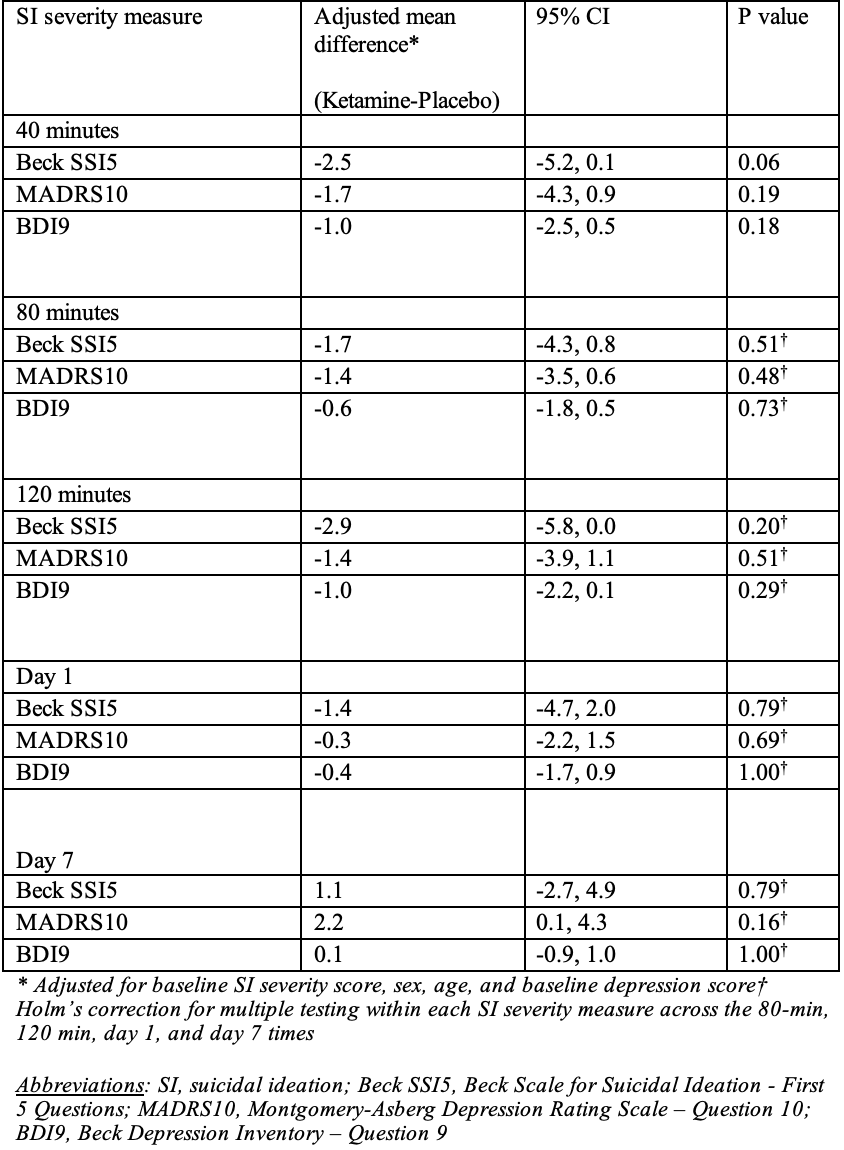
Table 1. Mean difference between groups in suicidal ideation severity


