Neonatal General 4: Novel Technology and Therapies
Session: Neonatal General 4: Novel Technology and Therapies
804 - Accuracy of a Wireless Oxygen Saturation Monitor (SpO2) in the Neonatal Intensive Care Unit
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 804.3777
Eva B. Senechal, McGill University Faculty of Medicine and Health Sciences, Montreal, PQ, Canada; Daniel J. Radeschi, McGill University Faculty of Medicine and Health Sciences, Pointe Claire, PQ, Canada; Emily Jeanne, McGill University Faculty of Medicine and Health Sciences, Montreal, PQ, Canada; Vivian M.G.O. Azevedo, Universidade Federal de Uberlândia, Uberlandia, Minas Gerais, Brazil; Wissam Shalish, McGill University Faculty of Medicine and Health Sciences, Montreal, PQ, Canada; Robert E. Kearney, McGill University Faculty of Medicine and Health Sciences, Montreal, PQ, Canada; Guilherme Sant'Anna, McGill University, Montreal, PQ, Canada

Eva B. Senechal, BSc. (she/her/hers)
PhD. Candidate
McGill University Faculty of Medicine and Health Sciences
Montreal, Quebec, Canada
Presenting Author(s)
Background: In the neonatal intensive care unit (NICU), oxygen saturation (SpO2) monitoring relies on wired sensors connected to bedside monitors. This system can lead to pressure sores, tangled wires, complicates nursing care, and interferes with parental bonding. A new sensor was identified as a potential technology for wireless SpO2 monitoring in the NICU.
Objective: To evaluate the accuracy of the Nonin wireless sensor for SpO2 monitoring in the NICU compared to the standard wired system.
Design/Methods: After parental consent was obtained, NICU patients had SpO2 monitored 8h/day over four days with both the Nonin WristOx2™ 3150 wireless sensor (Nonin Medical Inc., US) and RD SET® NeoPt standard wired sensors (Massimo, USA). Synchronized vital sign data from the wired and wireless sensors were recorded with a Biosensor Data Aggregation and Synchronization system (BioDaSh) developed for the study. To assess performance of the wireless sensor, the coverage (fraction of recording time for which vital sign values were available) was determined. Then for periods where both wireless and wired signals were available, sample to sample agreement was assessed by computing the bias, 95% limits of agreement (LoA), and variance accounted for (vaf) (Figure 2). The clinical implications of agreement were further assessed using modified Clarke-Error Grids: one for patients on continuous positive airway pressure (CPAP) and another for those on room air (RA), reflecting varying SpO2 targets (Figures 3).
Results: 26 patients and 715.7 hours of data were collected. The median postmenstrual age and corrected weight of participants were 36.8 weeks (IQR: 33.8 – 38.7) and 2295 grams (IQR: 1702 – 2907). Seven patients were on CPAP, and 19 were on RA. Median coverages were 95% (IQR: 91 -98) for the wired sensor and 94 % (IQR:89-96) for wireless. When comparing periods where signal was available from both systems (623.6 hours), the median bias was 0.24 with 95% LoA=+/- 4.05 % sat/min, and vaf of 26.79 (IQR: 0 – 49.8). The Clarke error grids supported the general clinical validity of the wireless SpO2 sensor, with 94%, and 80% of points lying in Zone A, indicating the wireless device was within 5% of the wired and yielding the same clinical interpretation (Figure 3).
Conclusion(s): A new wireless SpO2 monitor demonstrated good accuracy and clinical reliability compared to standard wired systems, with comparable coverage to the reference, low bias and moderate agreement across all metrics. Ongoing work will further investigate causes for differences in SpO2 and examine the safety of this device for neonatal skin.
Figure 1. The new skin sensor wireless SpO2 device
 Legend: Figure A - Nonin WristOx2™ 3150 with 600CN attachment and Figure B - back of the device where AAA batteries to power the device are placed.
Legend: Figure A - Nonin WristOx2™ 3150 with 600CN attachment and Figure B - back of the device where AAA batteries to power the device are placed.Figure 2. Bland Altman and Regression Analysis for One Patient
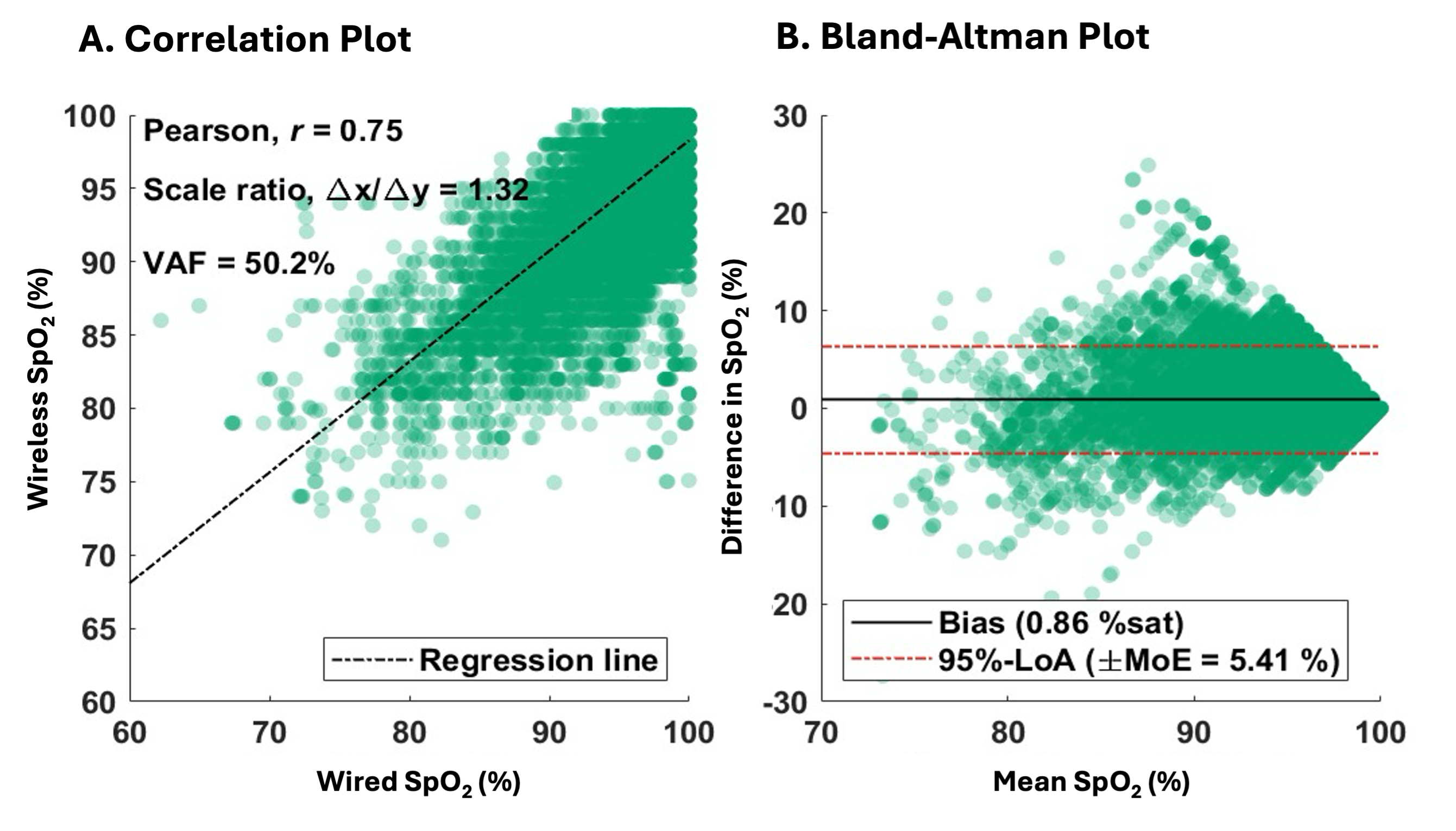 Legend: Panel A - linear regression of one patient with the pearson correlation coefficient, scale ration (slope), and variance accounted for (vaf). Panel B - Bland Altman analysis on the same patient with the bias, 95% limits of agreement (LoA) and margin of error (MoE).
Legend: Panel A - linear regression of one patient with the pearson correlation coefficient, scale ration (slope), and variance accounted for (vaf). Panel B - Bland Altman analysis on the same patient with the bias, 95% limits of agreement (LoA) and margin of error (MoE). Figure 3. Clarke Error Grid
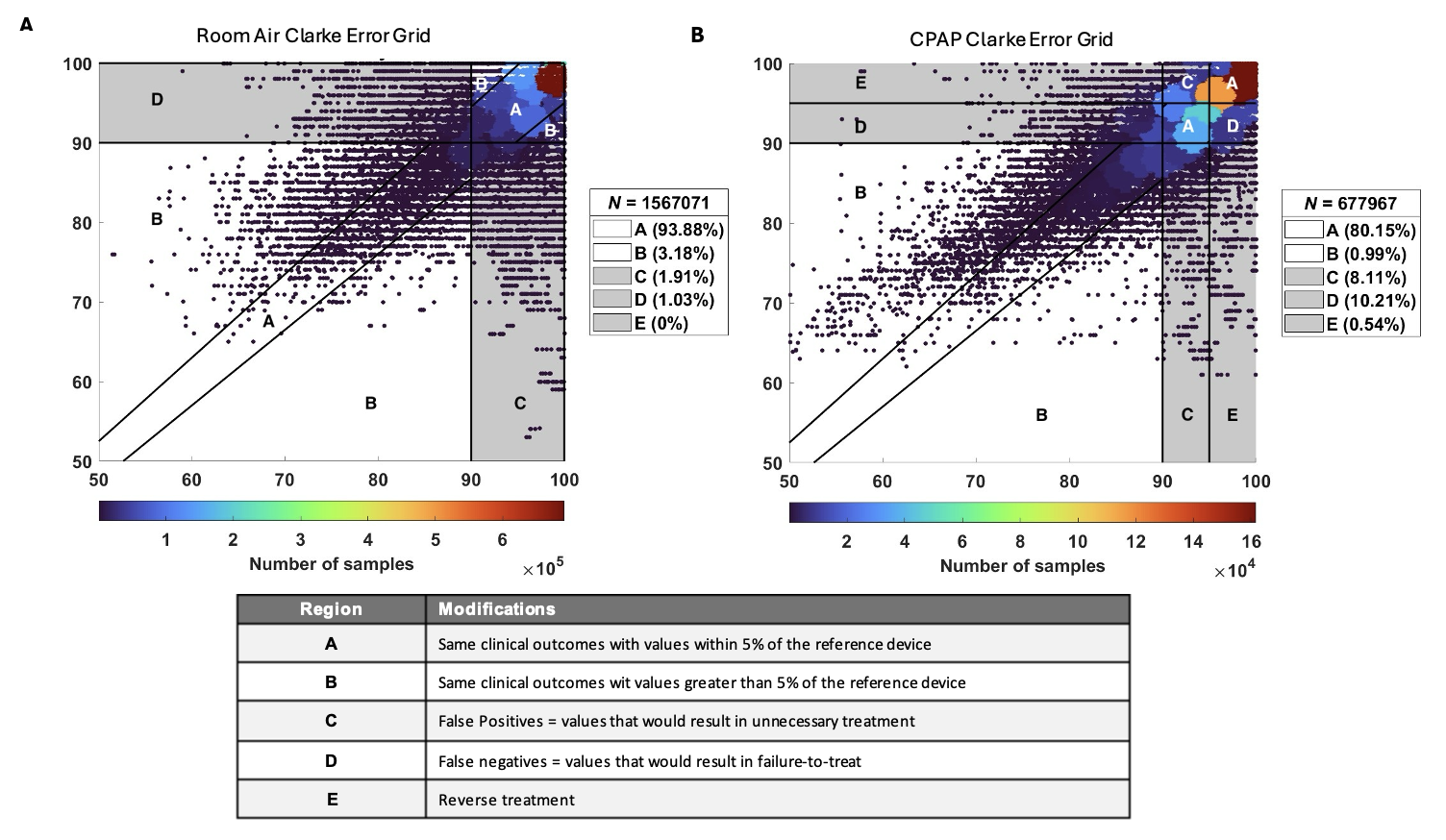 Legend: Panel A - Clarke Error Grid for patients on room air (RA, n=20). Panel B - Clarke Error Grid for patients on continuous positive airway pressure (CPAP, n=6). Any SpO2 value <90% was consider hypoxia and values >95% where considered hyperoxia. The percentage of data points assigned to each region is provided on the box at the right corner. Region definitions are provided in table below the figures.
Legend: Panel A - Clarke Error Grid for patients on room air (RA, n=20). Panel B - Clarke Error Grid for patients on continuous positive airway pressure (CPAP, n=6). Any SpO2 value <90% was consider hypoxia and values >95% where considered hyperoxia. The percentage of data points assigned to each region is provided on the box at the right corner. Region definitions are provided in table below the figures. 
