Global Neonatal & Children's Health 1
Session: Global Neonatal & Children's Health 1
727 - Antimicrobial-resistant bacteria from post-mortem culture isolates in Kenya: An emerging threat
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 727.3762
Jim S. Katieno, KEMRI, Kisumu, Nyanza, Kenya; Shirley Lidechi, Kenya Medical Research Institute, Kisumu, Kenya, Kisumu, Nyanza, Kenya; Zachary J.. Madewell, CDC, San Juan, N/A, Puerto Rico; Beth A. Tippett Barr, Nyanja Health Research Institute, Salima, Salima, Malawi; Dorothy Atieno. Odindo, kenya medical research institute, Kisumu, Nyanza, Kenya; Edwin Kiplagat. Kiplelgo, Kenya Medical Research Institute, Kabarnet, Rift Valley, Kenya; Clayton ONDU. Onyango, CDC-Kenya, Kisumu, Nyanza, Kenya; Kitiezo Aggrey. Igunza, Kenya Medical Research Institute, Kisumu, Nyanza, Kenya; Richard Omore, Kenya Medical Research Institute (KEMRI), Kisumu, Nyanza, Kenya; Victor Akelo, Kemri, Kisumu, Nyanza, Kenya; Naomi Lucchi, Centers for Disease Control and Prevention-Kenya, Nairobi, Nairobi Area, Kenya; Chris A. Rees, Emory University School of Medicine, Atlanta, GA, United States; Kephas Otieno, Kenya Medical Research Institute, Kisumu, Nyanza, Kenya
- CR
Chris A. Rees, MD, MPH
Assistant Professor
Emory University School of Medicine
Atlanta, Georgia, United States
Presenting Author(s)
Background: Antimicrobial resistance (AMR) is a major global public health challenge with an estimated 4.9 million deaths per year, disproportionately affecting children in low- and middle-income countries.
Objective: We describe AMR patterns in blood and cerebrospinal fluid (CSF) culture isolates among deceased children aged < 5 years enrolled in the Child Health and Mortality Prevention Surveillance (CHAMPS) in Western Kenya.
Design/Methods: The cross-sectional CHAMPS study of community- and facility-based deaths was conducted in two sites in Western Kenya (Karemo in Siaya County and Manyatta in Kisumu County) from January 2021 to December 2023. Deaths among children aged < 5 years were screened for eligibility through community and hospital surveillance. Extensive postmortem testing was done to determine causes of death and a subset of Gram-negative bacteria were tested against 12 antimicrobial drugs using the Kirby-Bauer Disk Diffusion method. Zones of bacterial inhibition were recorded, and isolates were grouped as susceptible, resistant, or intermediate to each antibiotic according to the 2023 Clinical and Laboratory Standards Institute guidelines.
Results: Of 634 cases enrolled within the period, 190 (30.0%) were stillbirths, 179 (28.2%) neonatal deaths, and 265 (41.8%) 1-59-month-olds. Escherichia coli and Klebsiella pneumoniae were the most common pathogens identified. Of these, 150 (23.7%) had blood culture and 34 (5.4%) had cerebrospinal fluid isolates assessed for antimicrobial resistance. Forty-eight (32.0%) E. coli and 39 (27.5%) K. pneumoniae blood culture isolates and 16 (47.1) E. coli and 10 (29.4%) K. pneumoniae CSF isolates were successfully assessed. A range of resistance to the 12 antibiotics tested was observed in both blood culture and CSF isolates of E. coli and K. pneumoniae including resistance to ceftriaxone (Third-generation cephalosporin antibiotic) and meropenem (carbapenem-class antibiotic) (Figure 1 and 2). Resistance to Amoxicillin Clavulanic acid and Ceftriaxone was greatest among children and neonates (Figure 3).
Conclusion(s): Resistance to commonly used and recommended antibiotics (including third-generation cephalosporin and carbapenem-class antibiotics) was common in this study population with some isolates showing resistance to multiple drugs. This could hamper treatment options in this region and may contribute to sustained high mortality rates. Continuous monitoring of antimicrobial resistance to inform strategies for antimicrobial stewardship programs and improving national efforts to reduce antimicrobial resistance is crucial.
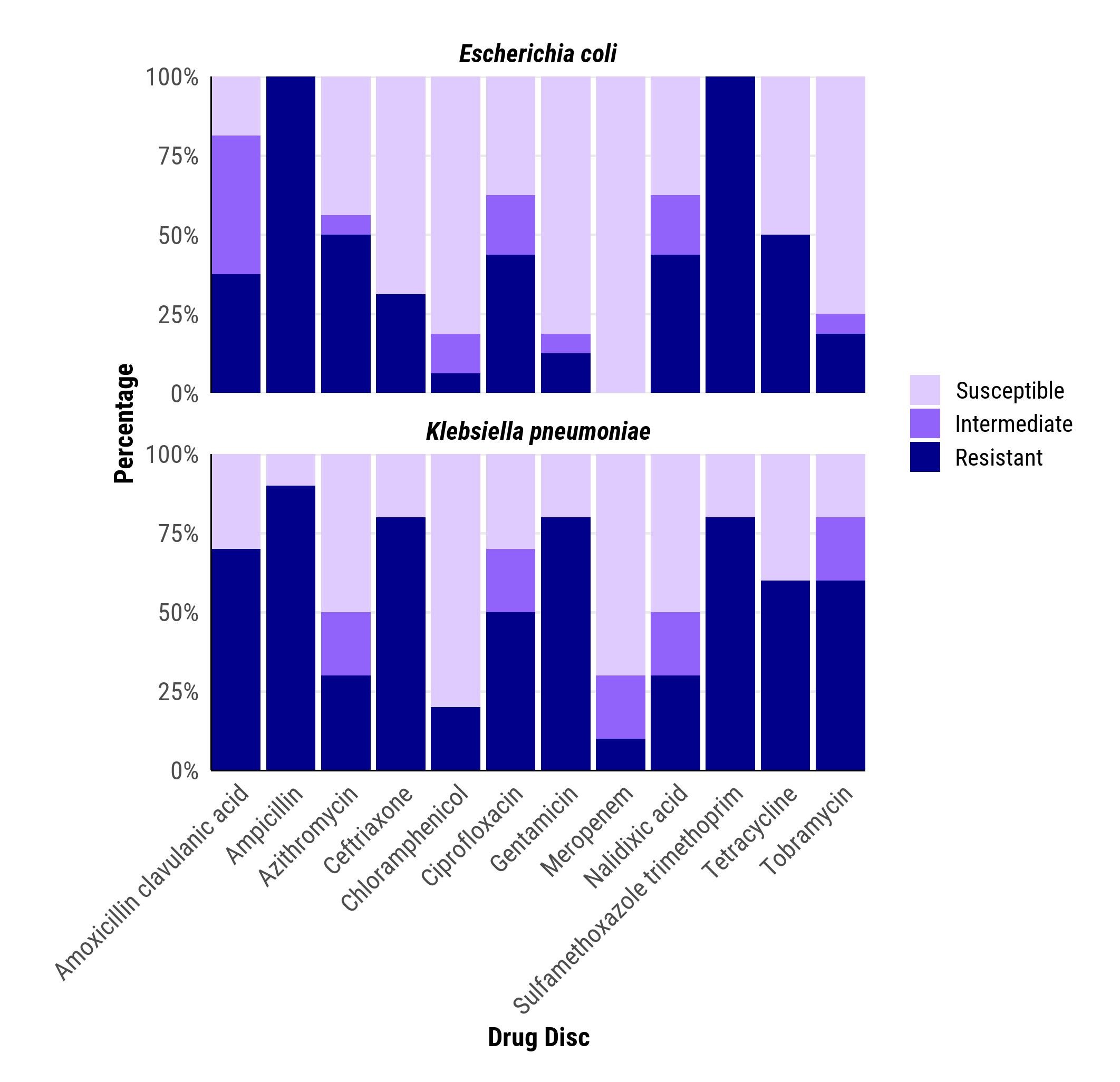
Fig. 1 Antimicrobial resistance patterns for Escherichia coli (n=48) and Klebsiella pneumoniae (n=39) isolated in blood cultures among deceased children aged <5 years in Western Kenya.
.jpg) Forty-eight (32.0%) blood cultures grew E. coli, of which 35.4% were resistant to ceftriaxone and 4.2% resistant to meropenem. Thirty-nine (27.5%) blood cultures grew K. pneumoniae; of which 51.3% were resistant to ceftriaxone and 10.3% had intermediate resistance to meropenem.
Forty-eight (32.0%) blood cultures grew E. coli, of which 35.4% were resistant to ceftriaxone and 4.2% resistant to meropenem. Thirty-nine (27.5%) blood cultures grew K. pneumoniae; of which 51.3% were resistant to ceftriaxone and 10.3% had intermediate resistance to meropenem.Fig.2 Antimicrobial resistance patterns for Escherichia coli (n=16) and Klebsiella pneumoniae (n=10) isolated from CSF in deceased children aged <5 years in Western Kenya
 Sixteen (47.1%) of CSF cerebrospinal fluid isolates had E. coli, all of which were susceptible to meropenem, and 5 (31.2%) were resistant to ceftriaxone. Ten (29.4%) CSF cultures grew K. pneumoniae, of which 8 (80.0%) were resistant to ceftriaxone.
Sixteen (47.1%) of CSF cerebrospinal fluid isolates had E. coli, all of which were susceptible to meropenem, and 5 (31.2%) were resistant to ceftriaxone. Ten (29.4%) CSF cultures grew K. pneumoniae, of which 8 (80.0%) were resistant to ceftriaxone.Fig. 3 Antimicrobial resistance patterns of Escherichia coli and Klebsiella pneumoniae blood culture isolates stratified by age
.jpg) Antimicrobial resistance to Amoxicillin Clavulanic acid and Ceftriaxone(commonly used drugs) was greatest among neonates and 1-59 month-old children.
Antimicrobial resistance to Amoxicillin Clavulanic acid and Ceftriaxone(commonly used drugs) was greatest among neonates and 1-59 month-old children.
