Immunizations/Delivery 1
Session: Immunizations/Delivery 1
666 - Using behavioral science to address pediatric vaccine hesitancy: Results from a pilot randomized control trial
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 666.6628
Grace W. Ryan, University of Massachusetts Medical School, Worcester, MA, United States; Princilla A. Minkah, University of Massachusetts Medical School, Wocrester, MA, United States; Jillian Bongiorni, Baystate Children's Hospital, Springfield, MA, United States; Christine Frisard, University of Massachusetts Medical School, Worcester, MA, United States; Milagros C.. Rosal, UMass Chan Medical School, Worcester, MA, United States; Stephenie C. Lemon, UMass Chan Medical School, Worcester, MA, United States

Grace Ryan, PhD, MPH (she/her/hers)
Assistant Professor
University of Massachusetts Medical School
Worcester, Massachusetts, United States
Presenting Author(s)
Background: Vaccination rates for COVID-19 and influenza are low among children and adolescents, especially in underrepresented minority (URM) populations. We designed a multi-component clinic-based intervention to support vaccine uptake using evidence-based strategies and informed by local, qualitative research. Our intervention, CONFIDENCE, consists of a webinar training for providers, tailored poster campaign featuring clinic staff sharing personal motivations for vaccination, and parent-facing printed educational materials.
Objective: We conducted a pilot randomized control trial in clinics serving a high percentage of URM populations in Massachusetts to assess preliminary effectiveness and research feasibility of CONFIDENCE.
Design/Methods: We used a cluster randomized design with a waitlist comparison condition and recruited 8 clinics. Parents/caregivers of children ages 5 to 17 completed pre-post surveys that we used to assess changes in our primary outcomes, future intention to be vaccinated for COVID-19 and influenza, using generalized estimating equations with a random effect for clinic. We compared demographics between intervention and control clinics using chi-square tests.
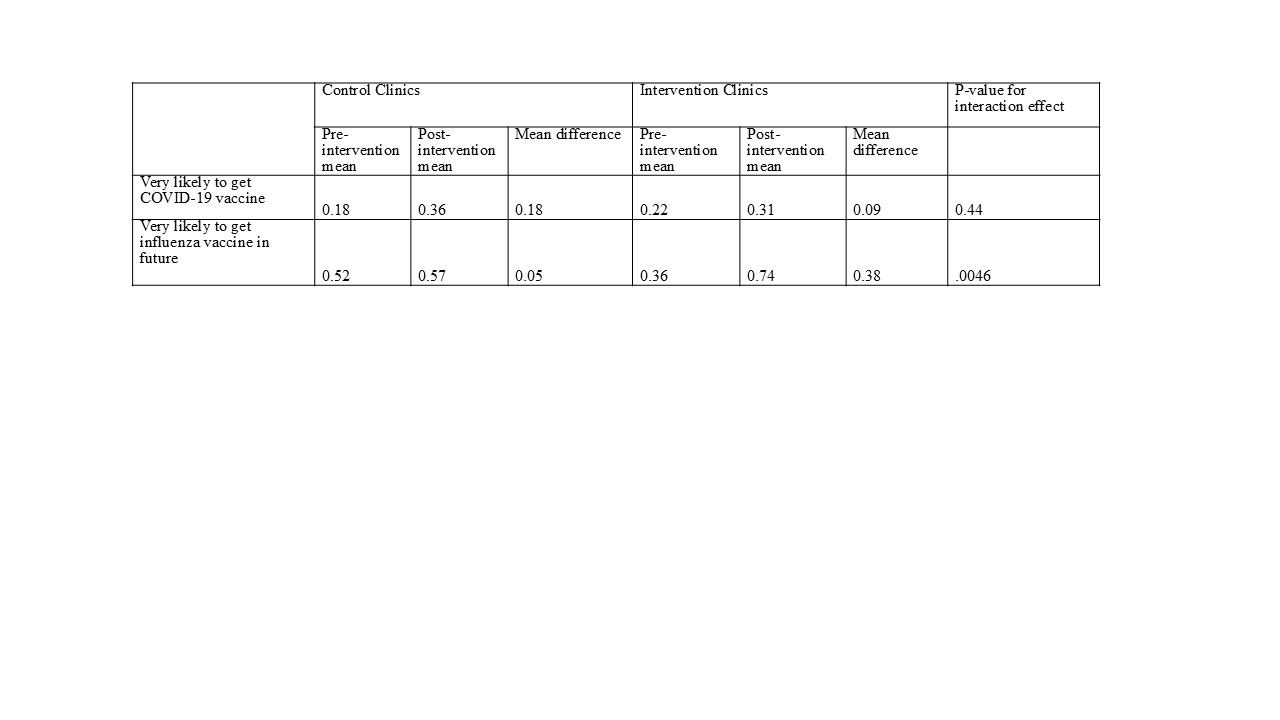
Results: All clinics successfully completed the intervention and data collection with 667 participants. There were no significant differences between clinics in terms of child gender (p=.57) and prior receipt of COVID-19 (p=.41) and influenza vaccines (p=.15). Children in the intervention clinic were younger (p=.0001) and a greater percentage were identified as Hispanic/Latino (p <.0001). Overall, high levels of parental vaccine hesitancy were observed, with nearly half of parents reporting their child had no prior COVID-19 vaccines. We observed significant improvement in influenza vaccine intention (intervention: 38% increase; control: 5% increase, p=.0046). Both intervention and control clinics improved in COVID-19 vaccine intention (9% and 18%, respectively, p=.44).
Conclusion(s): We recruited and retained 8 clinics to participate in CONFIDENCE and achieved a high level of participation from all sites. We found a positive and significant intervention effect to increase intention to vaccinate for influenza and observed an increase in COVID-19 vaccine intentions in both intervention and control clinics. Our positive outcomes in spite of high levels of hesitancy suggests that CONFIDENCE holds promise for supporting pediatric clinics in increasing vaccination rates. Future work will include conducting a hybrid implementation-effectiveness trial and expanding CONFIDENCE to address hesitancy for other vaccines.
Table 1. Comparison of demographics between intervention and control clinics, CONFIDENCE trial
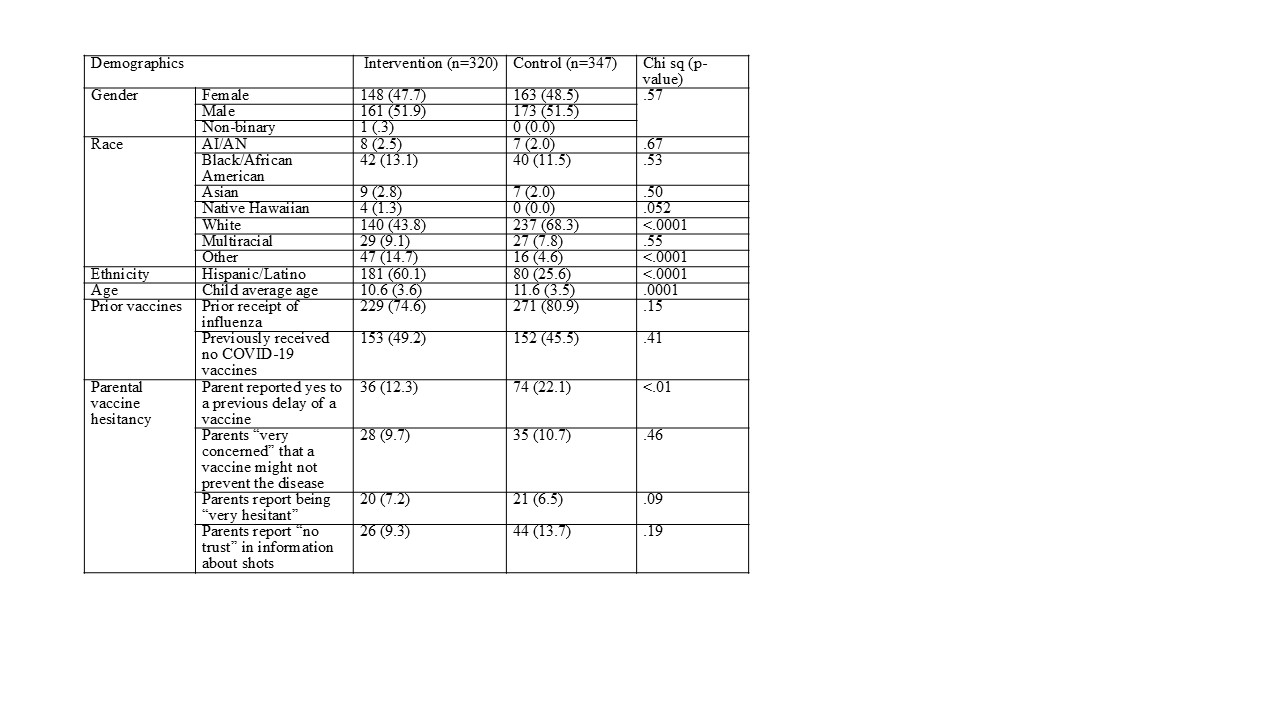
Table 2. Estimated means and mean differences for future intention to get vaccinated, CONFIDENCE trial