Neo-Perinatal Health Care Delivery: Epidemiology/Health Services Research 2
Session: Neo-Perinatal Health Care Delivery: Epidemiology/Health Services Research 2
358 - Nationwide cost-effectiveness analysis of human-derived milk fortifier using a microsimulation model of a US birth cohort.
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 358.5787
Brian King, Harvard Medical School/Beth Israel Deaconess Medical Center, Boston, MA, United States; John Zupancic, Beth Israel Deaconess Medical Center, Boston, MA, United States

Brian King, MD
Instructor
Harvard Medical School
Boston, Massachusetts, United States
Presenting Author(s)
Background: Human-derived milk fortifier (HDMF) use is increasing in US NICUs with the goal of reducing morbidity and mortality, however concerns persist regarding uncertainty in effectiveness and costs. Prior cost-effectiveness analyses have been confounded by effectiveness estimates derived from studies that combine the impact of fortifier type with donor milk. Two randomized trials are now published which have exclusively compared HDMF versus bovine milk fortifier (BMF), and cost-effectiveness can be evaluated using these published results.
Objective: To estimate the incremental cost-effectiveness ratio (ICER) of HDMF compared to BMF for extremely preterm infants using data from randomized trials exclusively studying fortifier type.
Design/Methods: Cost-effectiveness analysis from the societal perspective using a microsimulation model of a 1-year US birth cohort of infants born 23 to 27 weeks GA, comparing HDMF and BMF. The primary outcome was the incremental cost-effectiveness ratio (ICER), expressed as cost per quality-adjusted life year (QALY), estimated from the risk of moderate-severe NDI at 2-years. Effect estimates for a reduction in death, NEC and LOS with HDMF were derived from a meta-analysis of two published trials. Population distributions were derived from US natality data and GA-specific probabilities of death, NEC, late-onset sepsis and NDI were derived from published sources. Costs reported are in 2024 USD, and the willingness-to-pay (WTP) threshold was set at $50,000 per QALY.
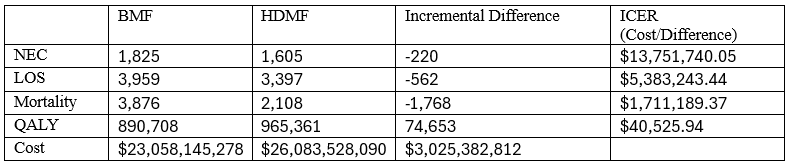
Results: Among an estimated 23,474 infants born 23 to 27 weeks GA annually in the US, HDMF may be associated with 220 fewer cases of NEC, 562 fewer cases of LOS and 1,768 fewer deaths (Table 1). The ICER for HDMF over BMF in this base case analysis was $40,526. Varying the effect estimates using the 95% confidence intervals from the two published trials across 10,000 iterations, the probability that HDMF was cost-effective was 63% (Figure 1). 2-way sensitivity analysis varying the odds of NEC and mortality suggest that cost-effectiveness is heavily dependent on the estimated reduction in mortality, with BMF showing favorability when the OR for mortality with HDMF is >0.85 (Figure 3).
Conclusion(s): Based on currently available evidence from randomized trials, HDMF may be cost-effectiveness across a range of WTP thresholds that are acceptable in the USA. Cost-effectiveness is primarily dependent on a reduction in mortality, for which the confidence interval from the two available clinical trials is very wide. A future trial, powered for a reduction in NEC and/or mortality, would improve the confidence in these estimates.
Table 1. Reduction in included outcomes and ICER for each outcome for the base case analysis among a theoretical annual US cohort of infants born 23 to 27 weeks gestational age (n = 23,474).
 Base case analysis used the point estimates for risk ratios (RR) from a meta-analysis of two published randomized trials (Optimom and N-Forte), which were as follows: NEC 0.89 (95% CI 0.41 to 1.97), Late-onset sepsis 0.86 (95% CI 0.41 to 1.77) and Mortality 0.57 (95% CI 0.27 to 1.22).
Base case analysis used the point estimates for risk ratios (RR) from a meta-analysis of two published randomized trials (Optimom and N-Forte), which were as follows: NEC 0.89 (95% CI 0.41 to 1.97), Late-onset sepsis 0.86 (95% CI 0.41 to 1.77) and Mortality 0.57 (95% CI 0.27 to 1.22).Figure 1. Probabilistic sensitivity analysis of an estimated annual US birth cohort.
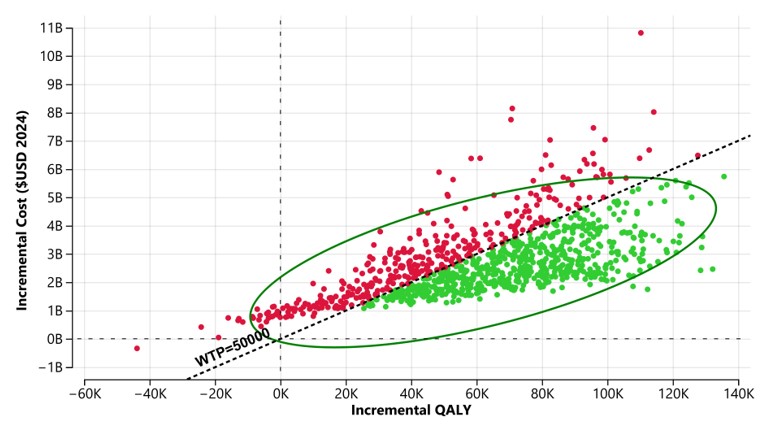 Probabilistic sensitivity analysis over 10,000 iterations which vary the effect estimates for reduction in NEC, LOS and mortality from HDMF across the 95% confidence interval from a meta-analysis of two randomized controlled trials. Green dots indicate iterations that were cost-effective with a willingness-to-pay threshold of 50,000 per QALY. Ellipses represents 95% of the iterations.
Probabilistic sensitivity analysis over 10,000 iterations which vary the effect estimates for reduction in NEC, LOS and mortality from HDMF across the 95% confidence interval from a meta-analysis of two randomized controlled trials. Green dots indicate iterations that were cost-effective with a willingness-to-pay threshold of 50,000 per QALY. Ellipses represents 95% of the iterations.Figure 2. 2-way sensitivity analysis simultaneously varying the odds of mortality and NEC with HDMF.
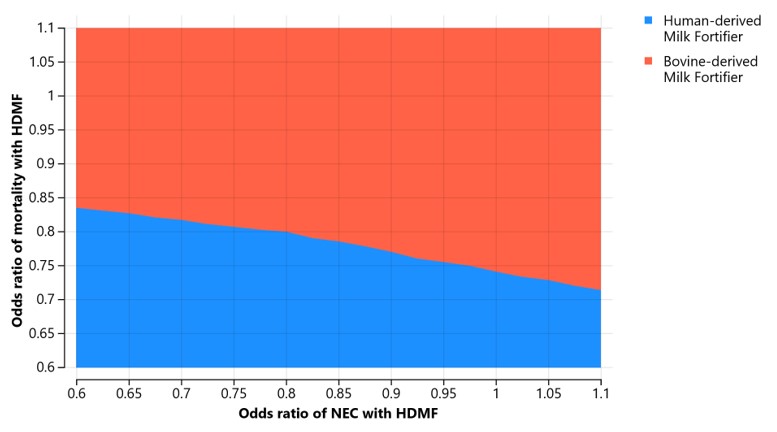 Color indicates which option is cost-effective using a WTP of $50,000 per QALY.
Color indicates which option is cost-effective using a WTP of $50,000 per QALY.
