Quality Improvement/Patient Safety 5
Session: Quality Improvement/Patient Safety 5
468 - A Pediatric Intensive Care Unit Quality Improvement Initiative to Optimize and Standardize Management of Ventilator-Associated Tracheitis
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 468.3691
Jesse A. Honig, Cohen Children's Medical Center, New Hyde Park, NY, United States; Evin S. Feldman, Cohen Children's Medical Center, White Plains, NY, United States; Denise E. Iacono, Cohen Children's Medical Center, New Hyde Park, NY, United States; Todd Sweberg, Cohen Children's Medical Center, New Hyde Park, NY, United States; Mundeep Kainth, Northwell Health, New Hyde Park, NY, United States

Jesse A. Honig, MD (he/him/his)
Chief Resident
Cohen Children's Medical Center
New Hyde Park, New York, United States
Presenting Author(s)
Background: Ventilator-associated tracheitis (VAT) represents a large bacterial and inflammatory burden in the lower respiratory tract of patients with artificial airways. It is among the most common diagnoses treated in the pediatric intensive care unit (PICU). Lack of consensus on how to diagnose or treat VAT has resulted in a prolonged duration of antibiotics and an overuse of tracheal aspirate cultures (TAC). Creating a VAT standard of care remains an area of significant interest as there is a relative paucity of quality improvement projects in this area and new evidence on how to optimize VAT diagnosis and management.
Objective: To improve VAT evaluation and management, we aimed to 1) increase the proportion of VAT treated for three days from 19% to 50% and 2) decrease the number of TAC sent per 100 endotracheal tube (ETT)/tracheostomy days by 25%.
Design/Methods: We launched a quality improvement initiative in a single urban tertiary care center. New institutional guidelines implemented in December 2023 provided standardized clinical criteria for obtaining TAC, microbiologic criteria for VAT diagnosis, and standardized VAT treatment duration from five to three days (Figure 1). Key stakeholders were engaged in departmental meetings providing education of updated recommendations. The study spanned 23 months pre-intervention and 8 months post-intervention. Primary outcome measures included proportion of three-day antibiotic courses and TAC sent per 100 ETT/tracheostomy days. Secondary analysis included antibiotic utilization data for VAT. Balancing measures included PICU mortality rate, number of ventilator-associated events (VAEs), ventilator days per month, and total PICU length of stay (LOS).
Results: There were 115 cases of VAT pre-intervention and 33 cases post-intervention. The proportion of VAT treated for 3 days increased from 19.1 to 81.8%. Antibiotic days of therapy for VAT per 1000 patient days also decreased from 30.1 to 18.9 (p < 0.01). There was no significant change in monthly median number of TAC sent per 100 ETT/tracheostomy days (77.8 pre-intervention vs 68.6 post-intervention, p=0.54) (Figure 2). Between the pre and post periods, there were no associated increases in PICU mortality rate (1.9% vs 2.0%, p=0.27), median monthly VAEs (0 vs 0, p=0.65), ventilator days per month (288.0 vs 331.0, p=0.99), or PICU LOS (4.8 vs 5.0 days, p=0.87).
Conclusion(s): Implementation of updated standardized VAT guidelines in critically ill children safely and effectively shortened antibiotic duration and utilization for VAT, but reducing the use of TAC remains a more challenging goal.
Figure 1: Updated guidelines on the diagnosis and management of ventilator associated tracheitis.
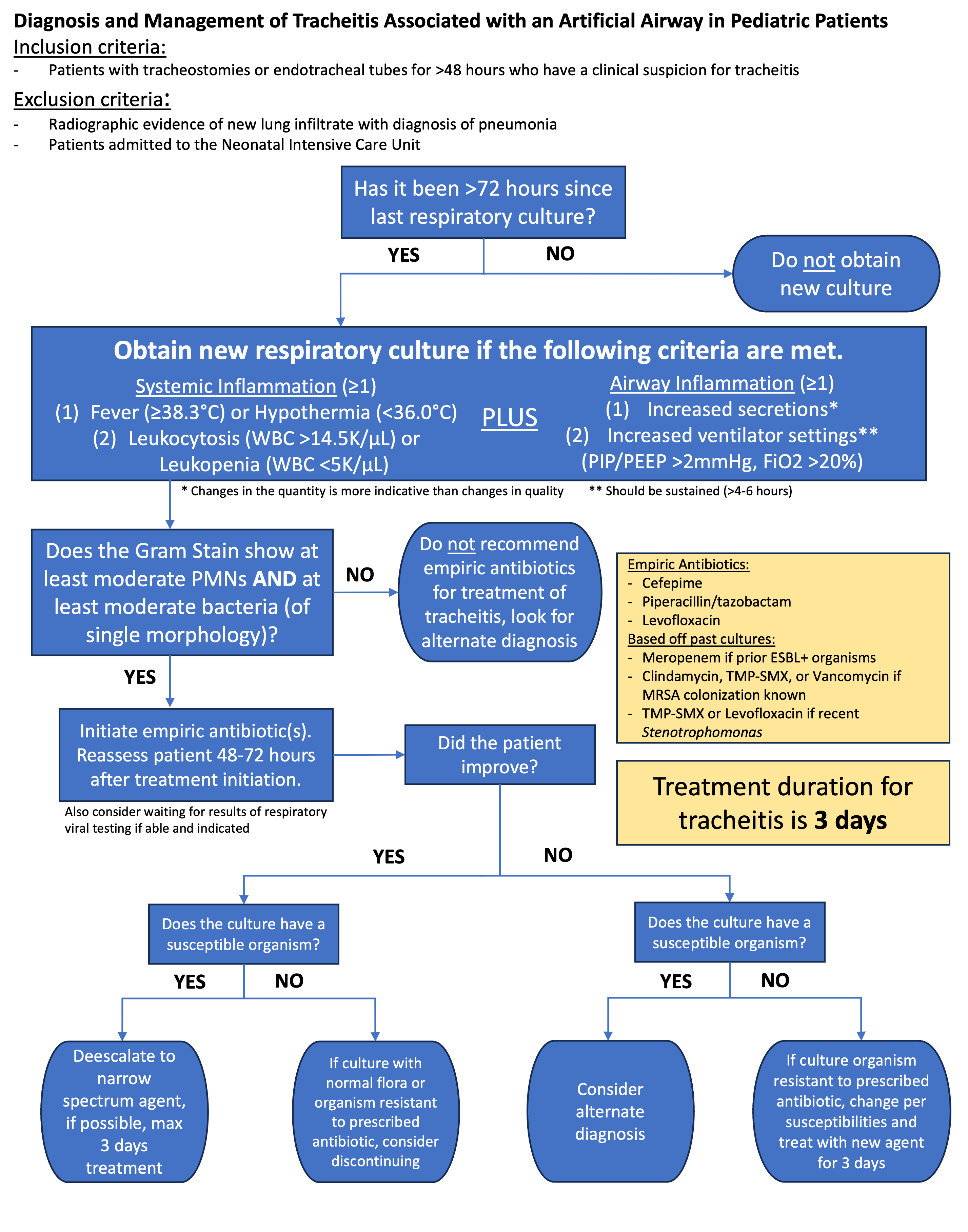
Figure 2: Outcome Measures
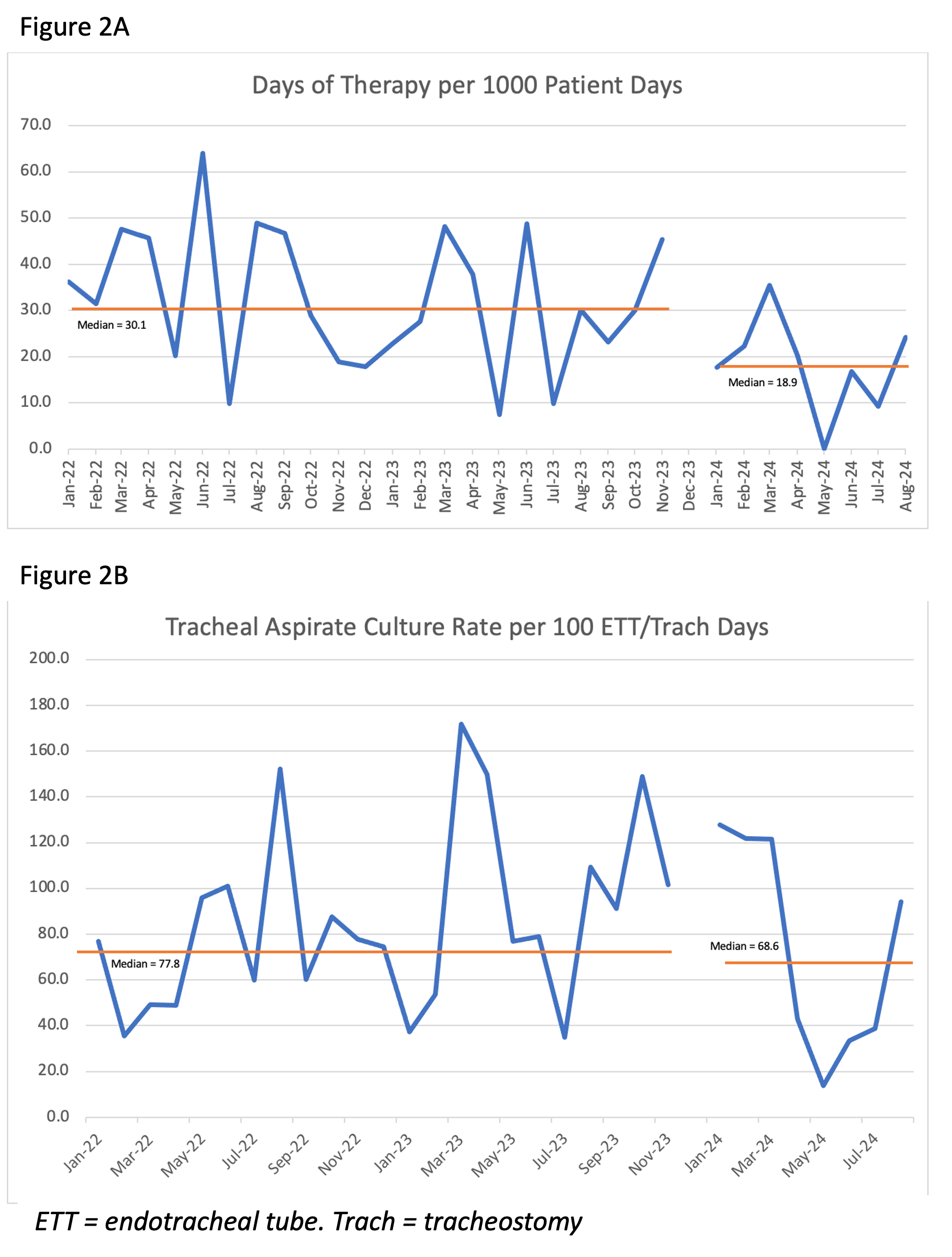 Figure 2A: Antibiotic days of therapy for ventilator associated tracheitis
Figure 2A: Antibiotic days of therapy for ventilator associated tracheitisFigure 2B: Median tracheal aspirate culture rate


