Quality Improvement/Patient Safety 6
Session: Quality Improvement/Patient Safety 6
485 - Developing a Wholistic Clinical Dataset for Duchenne Muscular Dystrophy
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 485.4155
Dax Bourcier, Dalhousie University, Dartmouth, NS, Canada; Victoria Hodgkinson, University of Calgary, Calgary, AB, Canada; Adrienna Dyck, University of Calgary, Calgary, AB, Canada; Eric J. Morden, Dalhousie University Faculty of Medicine, Oakville, ON, Canada; Jordan Sheriko, IWK Health/Dalhousie University, Halifax, NS, Canada

Dax Bourcier, MD, MSc (he/him/his)
Resident
Dalhousie University
Dartmouth, Nova Scotia, Canada
Presenting Author(s)
Background: Duchenne muscular dystrophy (DMD) is a neuromuscular disease that causes progressive muscle wasting. The Canadian Neuromuscular Disease Registry (CNDR) is a disease registry that collects prospective data from 38 clinics in Canada. The current CNDR-DMD module collects data focused on body structure and function; however, the International Classification of Functioning, Disability, and Health (ICF) – developed by the World Health Organization (WHO) – emphasizes that disability is multidimensional and involves four domains: body functions, activities and participation, body structures, and environmental factors.
Objective: Our objective was to develop comprehensive and brief ICF core sets for DMD and to link these to the CNDR-DMD module.
Design/Methods: A four-phased descriptive study was conducted (Fig 1). The process was compliant with WHO guidelines and co-developed with patient and parent representatives. The first phase was a literature review to identify existing ICF core sets relevant to DMD. The second phase was to develop an ICF comprehensive core set. Clinically relevant categories were identified, reviewed, and condensed using a modified Delphi method by a patient and parent committee and a health-care provider committee. In the third phase, a wholistic ICF brief core set was developed. This process included multi-stakeholder consensus meetings, ranking surveys, and evaluation of clinical feasibility. The fourth phase was to link the new ICF core sets to the CNDR-DMD registry to identify gaps in current data collection where new measures and outcomes can be added or piloted.
Results: The literature review identified an existing ICF set for DMD that only included body functions, and an ICF set for neuromuscular disorders. Our ICF core sets for DMD included 222 categories for the comprehensive set, and 40 and 43 categories for ambulatory and non-ambulatory DMD brief core sets, respectively (Figs 2-3). The activities & participation and environmental factors had the biggest gaps in the CNDR-DMD subset after the linking process.
Conclusion(s): This study produced the first wholistic ICF core sets for DMD. The next steps are to identify and pilot outcome measures that align with the brief ICF core set for DMD for their inclusion in the CNDR registry to close existing gaps and promote standardized collection in participating neuromuscular clinics in Canada. The CNDR’s expanded DMD dataset will support robust real-world data collection for natural history studies, post-marketing surveillance of novel therapies, and planning of research that reflects priorities of patients and families affected by DMD.
Figure 1
 Four-phased methodology
Four-phased methodologyFigure 2
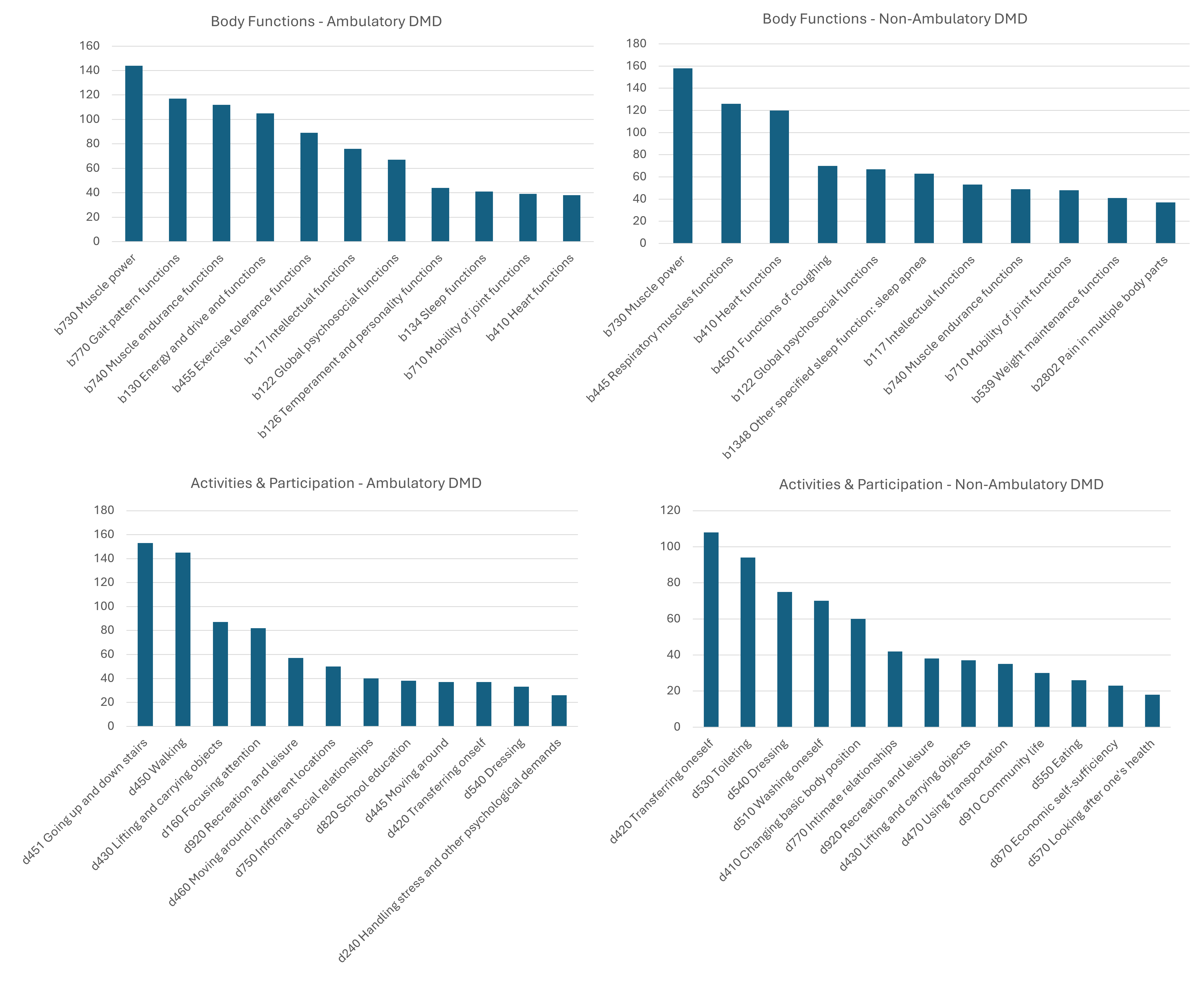 ICF Brief Core Set for DMD showing final weighed ranks for body functions and activities & participation categories for ambulatory and non-ambulatory DMD.
ICF Brief Core Set for DMD showing final weighed ranks for body functions and activities & participation categories for ambulatory and non-ambulatory DMD.Figure 3
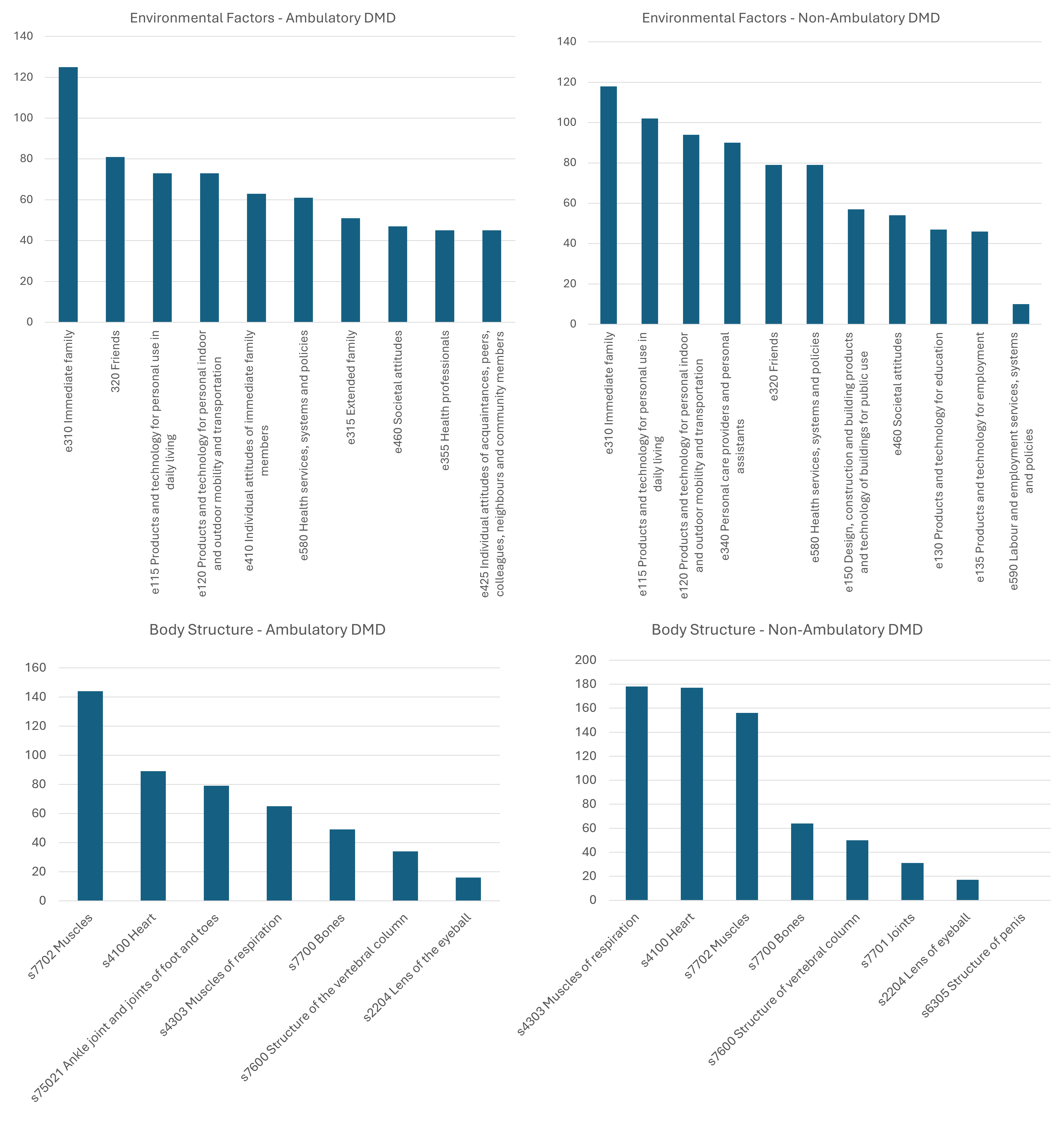 ICF Brief Core Set for DMD showing final weighed ranks for environmental factors and body structures categories for ambulatory and non-ambulatory DMD
ICF Brief Core Set for DMD showing final weighed ranks for environmental factors and body structures categories for ambulatory and non-ambulatory DMD 
