Clinical Bioethics
Session: Clinical Bioethics
577 - Development of a Dyadic Tool to Measure the Process of Shared Decision-Making in Pediatrics: The Survey of Pediatric Encounters About Kids (SPEAK)
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 577.4167
Elsa M. Ayala, Seattle Children's Hospital, Seattle, WA, United States; Heather Spielvogle, Seattle Children's, Seattle, WA, United States; Olivia Orr, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Akram Ibrahim, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Abril Beretta, Seattle Children's, seattle, WA, United States; Emily Kroshus-Havril, University of Washington School of Medicine, Seattle, WA, United States; Elliott M. Weiss, University of Washington, Seattle, WA, United States; Chuan Zhou, Seattle Children's Research Institute, Seattle, WA, United States; Seema K. Shah, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Douglas J.. Opel, Seattle Children's Research Institute, Seattle, WA, United States
.jpg)
Elsa M. Ayala, BS (she/her/hers)
Clinical Research Coordinator II
Seattle Children's Hospital
Seattle, Washington, United States
Presenting Author(s)
Background: Incorporating clinician and patient perspectives in the measurement of shared decision-making (SDM) is aligned with SDM’s inherently dyadic nature. There are no tools measuring SDM in pediatrics from multiple perspectives.
Objective: The objective of this study was to develop a dyadic instrument to measure SDM from the perspectives of both clinicians and parents of young children.
Design/Methods: We used a stepwise iterative approach to tool development beginning with de novo item generation and followed by augmentation of the item pool by adapting items from existing instruments. To facilitate item reduction, 3 parents and 3 SDM experts reviewed our preliminary items and rated their ability to capture SDM in pediatrics (with 1=not at all well and 4=very well); items with the lowest mean ratings were removed. To assess the preliminary tool’s face validity, usability, and item understandability, we then pretested it, revising it iteratively, with sequential cohorts of English-speaking parents and clinicians from two US children’s hospitals.
Results: We generated an initial list of 20 items for the parent- and clinician-versions of the dyadic tool. After review by parents and SDM experts, we cut 9 items and added one additional item for a total of 12 items. We pretested the preliminary tool with 31 clinicians (Table 1) and 30 parents (Table 2) across 3 sequential cohorts, resulting in several grammatical and syntactical revisions. In addition, based on pretest participant input, we (a) revised the tool to include a behavioral response scale (Not at all/A little/Somewhat/Mostly /A lot) to items, rather than a Likert Scale, to better align with the fact that items on the tool were describing behaviors; (b) broadened the wording on the tool to be inclusive of all decision encounters regardless of whether SDM was used in order to maximize the tool’s utility; and (c) made changes to the tool to reduce potential social desirability bias that may skew responses in the direction of indicating that a comprehensive form of SDM was used. The final tool contained 12 items and an optional free text item (Figure 1).
Conclusion(s): We have developed a usable preliminary dyadic tool for measuring the process of SDM in pediatrics that demonstrates face and content validity. This tool represents an important first step towards addressing the measurement for SDM in pediatrics. Evaluation of its psychometrics is needed.
Table 1. Demographics of Clinician Participants in Pretesting of Tool (N=31)
.jpg)
Table 2. Demographics of Parent Participants in Pretesting Tool (N=30)
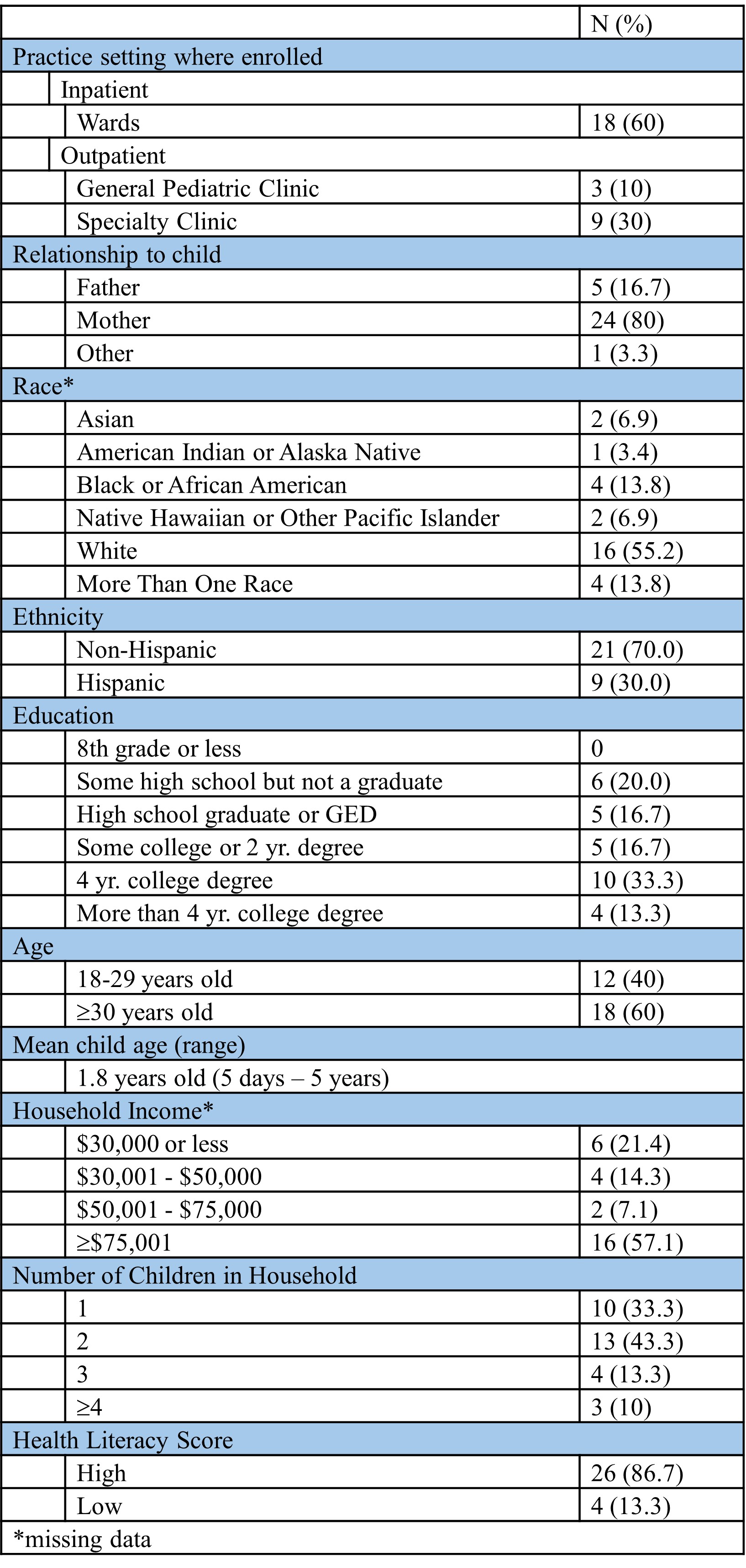
Figure 1. Final SPEAK tool


