Emergency Medicine 13
Session: Emergency Medicine 13
564 - Using Quality Improvement Methodology to Inform Safe Implementation of Propofol for Procedural Sedation
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 564.7014
Ali Wiersma, University of Colorado School of Medicine, Denver, CO, United States; Nikita Habermehl, Children's Hospital Colorado, Aurora, CO, United States; Stephanie Pennington, Children's Hospital Colorado, Denver, CO, United States; Tamara Garcia, Children's Hospital Colorado, Aurora, CO, United States; Mark G. Roback, University of Colorado School of Medicine, Denver, CO, United States

Alexandria Wiersma, MD
Assistant Professor, Pediatric Emergency Medicine
University of Colorado School of Medicine
Denver, Colorado, United States
Presenting Author(s)
Background: The safety of propofol for procedural sedation in the emergency department is well-established. Propofol provides deep sedation with rapid onset of action and return to baseline. Despite widespread use of propofol in many pediatric emergency departments (PED) across the country, our institution primarily uses ketamine for procedural sedation. As a result, there was variable knowledge by our PED staff regarding its safety, efficacy, and mechanism of action at the time it became available for use.
Objective: To address these needs and safely introduce propofol for procedural sedation in our PED, this Quality Improvement (QI) project was initiated. By offering propofol as a sedation option, we aimed to maintain or decrease the number of serious adverse events (SAE) associated with procedural sedation while reducing our median length of sedation from 45 min to less than 30 min by February 2025.
Design/Methods: A multidisciplinary QI team was formed to understand current process for procedural sedation, define scope for propofol use, and design and implement interventions needed for safe introduction. The team utilized knowledge from prior propofol experiences as well as a review of the current literature to determine the most appropriate scope and methods for use. The team then used a multi-modal education approach (lectures, simulation, and “train the trainer”) to ensure knowledge and comfort of all staff. Data was monitored throughout the implementation process, including length of sedation, length of stay (LOS), adverse events (AE), and SAE.
Results: In the 8 months following introduction of propofol, there were 651ketamine sedations and 40 propofol sedations completed in our PED. The median length of sedation for all sedations decreased from 45 min to 35 min. Notably, median length of sedation for propofol alone was 17.5 min. Total LOS decreased from 390 min to 362 min (338 min for propofol alone). There was no change in overall AE or SAE, which remained around 10% and < 1% respectively. There were no SAE reported in the propofol group.
Conclusion(s): Using QI methodology, we were able to safely introduce propofol as a tool for procedural sedation in our PED without any significant change in SAE. We demonstrated improvement in both total length of sedation and LOS, particularly for those patients that received propofol, indicating a value of time savings to both families and staff members. This QI project can provide a framework for implementation of a known, safe medication.
Figure 1. Run chart of length of sedation.
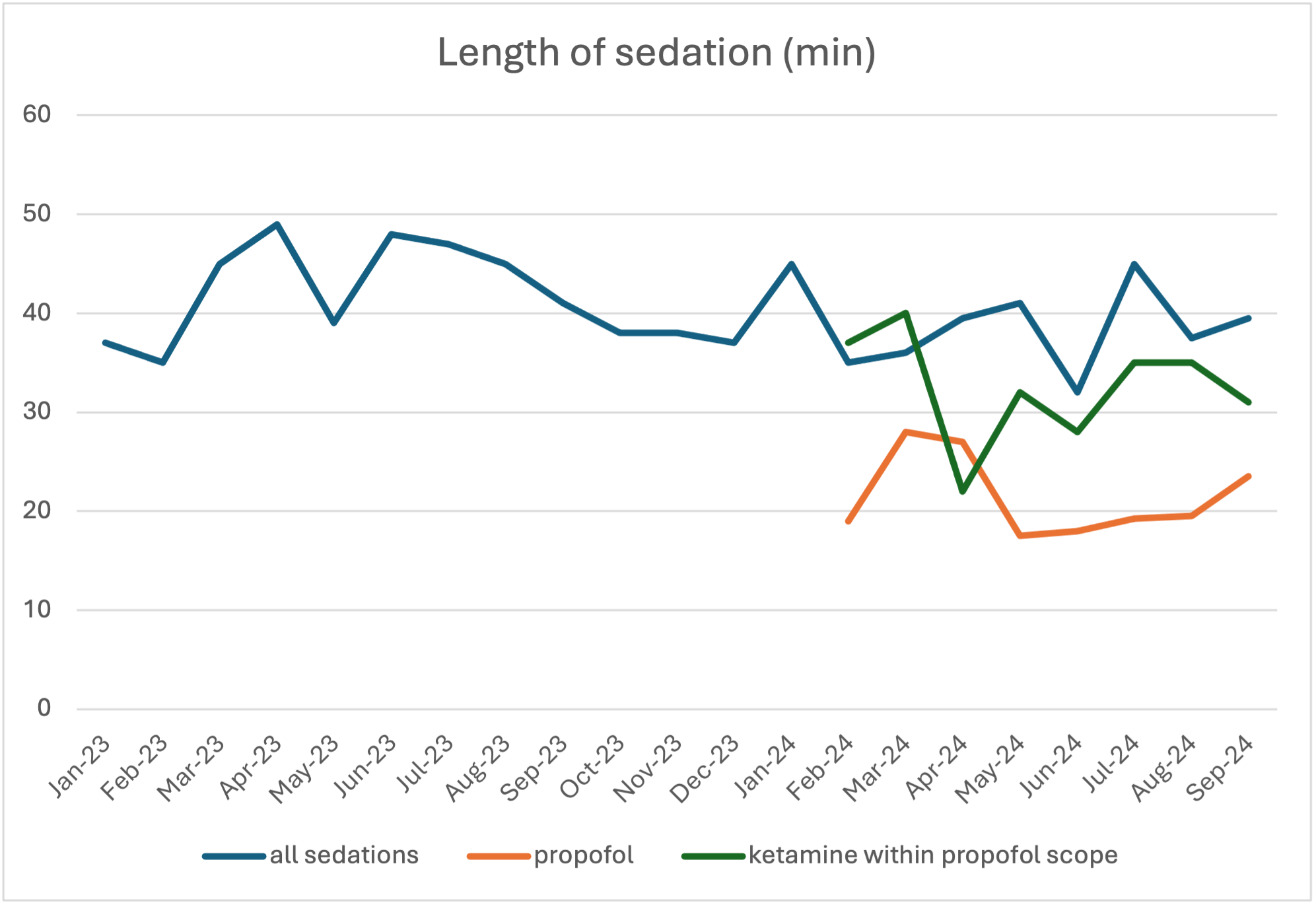 Comparing length of sedation (min) for all sedations, propofol sedations alone, and ketamine sedations within propofol scope
Comparing length of sedation (min) for all sedations, propofol sedations alone, and ketamine sedations within propofol scopeFigure 2: I Chart of length of sedation by month
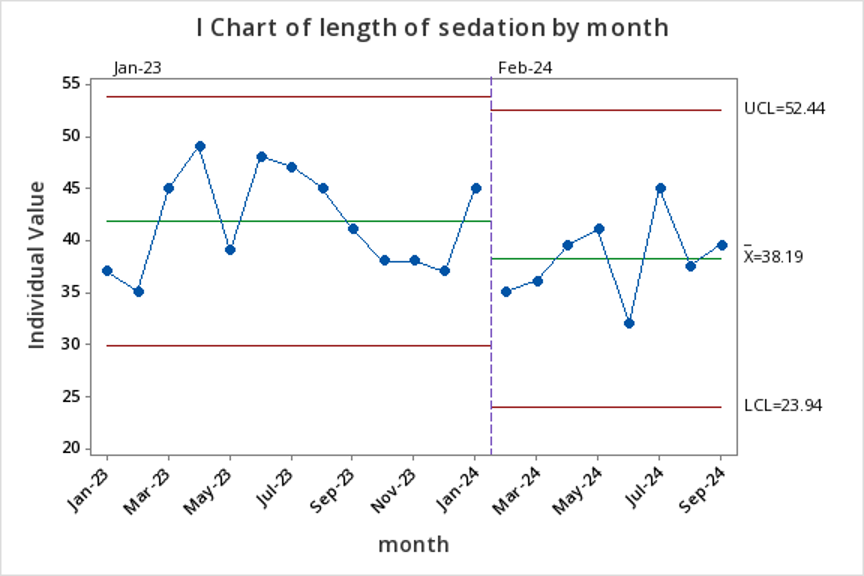 Pre and post intervention total length of sedation (all sedations)
Pre and post intervention total length of sedation (all sedations)Figure 3: Key Measures Table
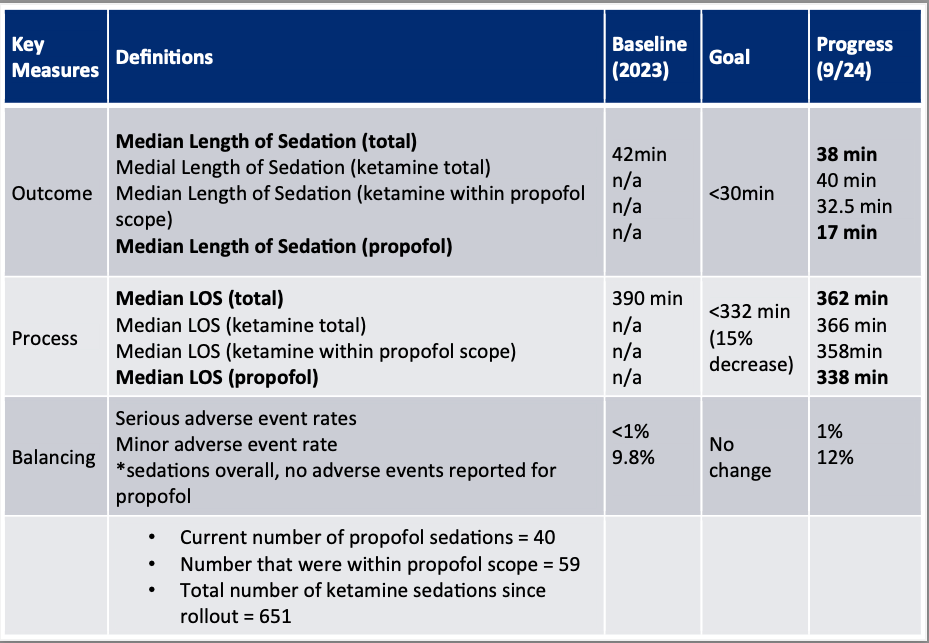
Figure 1. Run chart of length of sedation.
 Comparing length of sedation (min) for all sedations, propofol sedations alone, and ketamine sedations within propofol scope
Comparing length of sedation (min) for all sedations, propofol sedations alone, and ketamine sedations within propofol scopeFigure 2: I Chart of length of sedation by month
 Pre and post intervention total length of sedation (all sedations)
Pre and post intervention total length of sedation (all sedations)Figure 3: Key Measures Table



