Public Health & Prevention 3
Session: Public Health & Prevention 3
780 - Engaging Pediatric Subspecialists in the Regulatory Process to Reduce Added Sugars Intake
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 780.4662
Jang Lee, Stanford University School of Medicine, Stanford, CA, United States; Eimaan Anwar, Boston College Law School, Brighton, MA, United States; Alison Clayshulte, School of Nursing and Health Professions, University of San Francisco, Moraga, CA, United States; Lisa Chamberlain, Stanford University School of Medicine, Palo Alto, CA, United States; Janine Bruce, Stanford University School of Medicine, Stanford, CA, United States; Christina Hecht, University of California Nutrition Policy Institute, Oakland, CA, United States; Shweta Namjoshi, Stanford University School of Medicine, Palo Alto, CA, United States; Noelle Ebel, Lucile Packard Children's Hospital Stanford, Palo Alto, CA, United States; Anisha Patel, Stanford University School of Medicine, palo alto, CA, United States
- JL
Jang Lee, MPH (he/him/his)
Medical Student
Stanford University School of Medicine
Stanford, California, United States
Presenting Author(s)
Background: Excess consumption of added sugars negatively impacts child health. Despite policy efforts to reduce intake of added sugars, consumption in US children still exceeds recommended limits. In 2022, the White House announced a National Strategy on Hunger, Nutrition, and Health to reduce hunger and diet-related diseases by 2030. As part of this strategy, the Food and Drug Administration (FDA) organized a virtual public meeting and listening sessions and solicited public comment on strategies to reduce added sugars intake. This presented a unique opportunity to mobilize pediatric subspecialty healthcare providers to provide comments as they witness the downstream negative health impacts of added sugars intake in patients.
Objective: This case study highlights a model for pediatric subspecialists to submit public comments on important regulatory issues impacting child health.
Design/Methods: The pilot had four steps: 1) engage advocacy organizations and government relations 2) identify scholars with content expertise for evidence-based recommendations, 3) develop an efficient process, including template letters, for comment submission, and 4) promote the opportunity to provide comments.
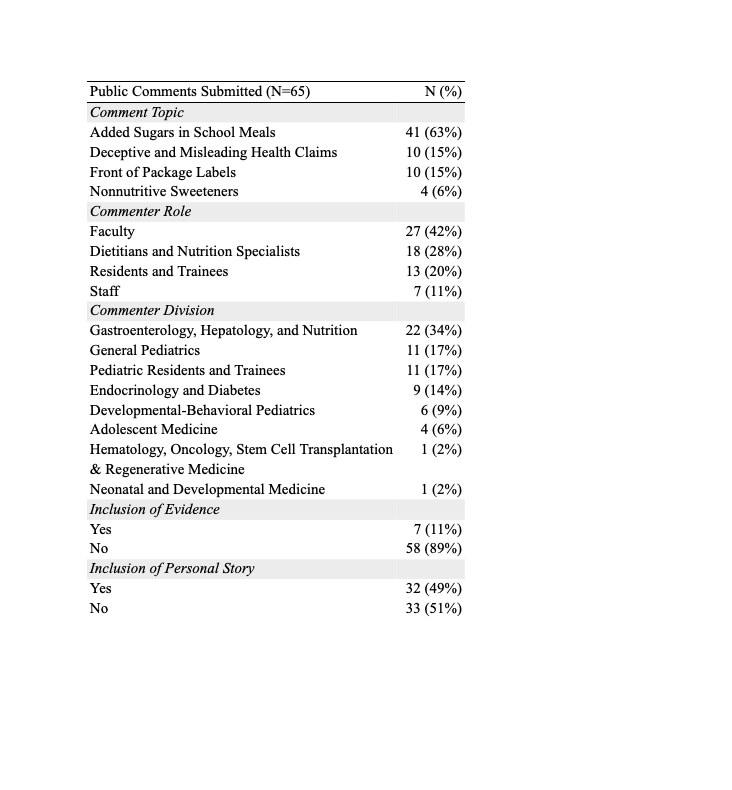
Results: Pediatric subspecialists participating in the pilot submitted 65 comments (16% of overall comments submitted to FDA). Comments focused on one of four pre-chosen strategies to reduce added sugars in the diet: added sugars in school meals, deceptive and misleading health claims, front-of-package labels, and non-nutritive sweeteners (Table 1). Nearly half of submitters incorporated a personal or patient story in the template letter that reflected diverse subspecialty perspectives (Table 2).
Conclusion(s): By leveraging the expertise of content experts and government relations, the pilot provided an effective model for engaging pediatric subspecialists in submitting FDA comments regarding strategies for reducing added sugars in the diet. Offering content drafted by experts with opportunity for personalization made it easy for pediatric subspecialists to provide evidence-based comments that included patient stories important to policymakers and the regulatory process. This pilot demonstrated that engaging pediatric subspecialists in the regulatory process is feasible.
Demographic Data of Public Comment Submissions for Food and Drug Administration's Strategies to Reduce Added Sugars Consumption in the United States (Docket No. FDA-2023-N-3849)
Illustrative Quotes Submitted by Subspeciality Pediatric Divisions for Food and Drug Administration's Strategies to Reduce Added Sugars Consumption in the United States (Docket No. FDA-2023-N-3849)
.png)
Example Template Letter on Added Sugars in School Meals
.jpg)
Demographic Data of Public Comment Submissions for Food and Drug Administration's Strategies to Reduce Added Sugars Consumption in the United States (Docket No. FDA-2023-N-3849)

Illustrative Quotes Submitted by Subspeciality Pediatric Divisions for Food and Drug Administration's Strategies to Reduce Added Sugars Consumption in the United States (Docket No. FDA-2023-N-3849)
.png)
Example Template Letter on Added Sugars in School Meals
.jpg)

