Neonatal Fetal Nutrition & Metabolism 4
Session: Neonatal Fetal Nutrition & Metabolism 4
645 - Modeling Neonatal Weight Gain with High-Fidelity Nutritional Data
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 645.5893
William King, Medical Predictive Science Corporation, Charlottesville, VA, United States; Gustave Falciglia, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
- WK
William King, MSEE (he/him/his)
CEO
Medical Predictive Science Corporation
Charlottesville, Virginia, United States
Presenting Author(s)
Background: Neonatologists lack automated data on nutrition intake to ensure adequate delivery and to balance various nutrition delivery practices with fluid intake, leading them to often fail to deliver the recommended intake with large deficits accruing during hospitalization. 50% of the 51,000 very low birth weight (VLBW, birth weight < 1500g) infants born in the US each year leave the NICU at a discharge weight < 10th percentile for their corrected, postmenstrual age (PMA). We hypothesize that presenting real-time energy, macronutrient, micronutrient, and fluid intake data alongside growth modelling will improve clinicians’ ability to deliver high quality neonatal nutrition and achieve optimal growth.
Objective: We sought to model growth as a function of high-fidelity electronic health record (EHR) data.
Design/Methods: From over 600 infants at the Lurie Children’s NICU, we extracted the complete data necessary to calculate growth, fluid intake, and nutrition orders, via a real-time HL7 messaging interface with the EHR. This provides necessary, real-time data granularity that was not available from archived data. We developed a natural language processing (NLP) algorithm which accurately links the approximately 1 million fluid intakes to nutritional orders. We further developed a second NLP algorithm that calculates energy content from the fluid intakes and nutritional orders. We modeled weight gain as a function of NLP-calculated energy intake along with other independent variables.
Results: In a multivariable generalized linear model, we found the following variables to each be significant independent predictors of weight gain: PMA, NLP-calculated daily energy intake per kg, pulse rate per kg, day from birth, birth weight, return from procedure, intake volume, output volume, and ratios and differences between the latter two. The modeled weight gain had a correlation coefficient of 0.56 compared with actual weight gain.
Conclusion(s): We found reasonable model performance to predict weight gain based on EHR parameters, and expect to improve model performance as we test additional factors including macronutrients, micronutrients, higher granularity ECG-derived heart rate, respiratory rate, ventilatory status, temperature, and effects of feeding and circadian rhythms.
Table 1.
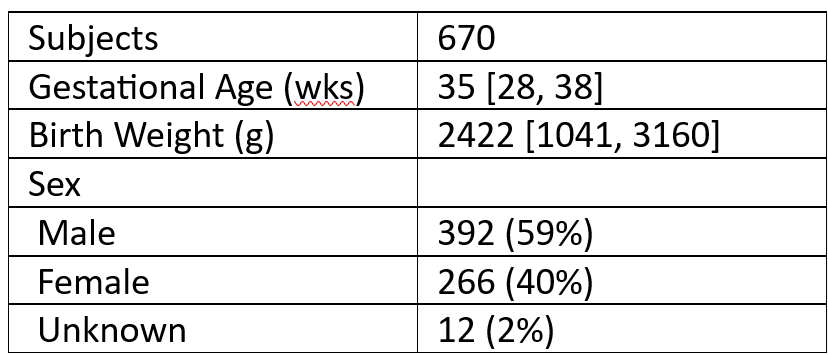 Patient demographics. Data are expressed as Median [IQR] or Count (Percent).
Patient demographics. Data are expressed as Median [IQR] or Count (Percent).Figure 1.
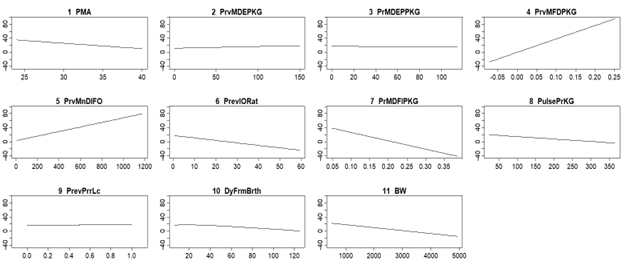 Model response to predictor variables. Y-axis is modeled weight gain per KG per day over the previous seven days. 1 PMA: Postmenstrual Age. 2 PrvMDEPKG: Previous Mean Daily Energy per KG. 3 PrMDEPPKG: Previous Mean Daily Parenteral Energy per KG. 4 PrvMFDPKG: Previous Mean Fluid I/O Difference per KG. 5 PrvMnDlFO: Previous Mean Daily Fluid Output. 6 PrevIORat: Previous I/O Ratio. 7 PrMDFIPerKG: Previous Mean Daily Fluid Intake per KG. 8 PulsePrPG: Pulse per KG. 9 PrevPrrLc: Previous Prior Location (e.g. surgery). 10 DyFrmBrth: Day From Birth. 11 BW: Birth weight.
Model response to predictor variables. Y-axis is modeled weight gain per KG per day over the previous seven days. 1 PMA: Postmenstrual Age. 2 PrvMDEPKG: Previous Mean Daily Energy per KG. 3 PrMDEPPKG: Previous Mean Daily Parenteral Energy per KG. 4 PrvMFDPKG: Previous Mean Fluid I/O Difference per KG. 5 PrvMnDlFO: Previous Mean Daily Fluid Output. 6 PrevIORat: Previous I/O Ratio. 7 PrMDFIPerKG: Previous Mean Daily Fluid Intake per KG. 8 PulsePrPG: Pulse per KG. 9 PrevPrrLc: Previous Prior Location (e.g. surgery). 10 DyFrmBrth: Day From Birth. 11 BW: Birth weight.Figure 2.
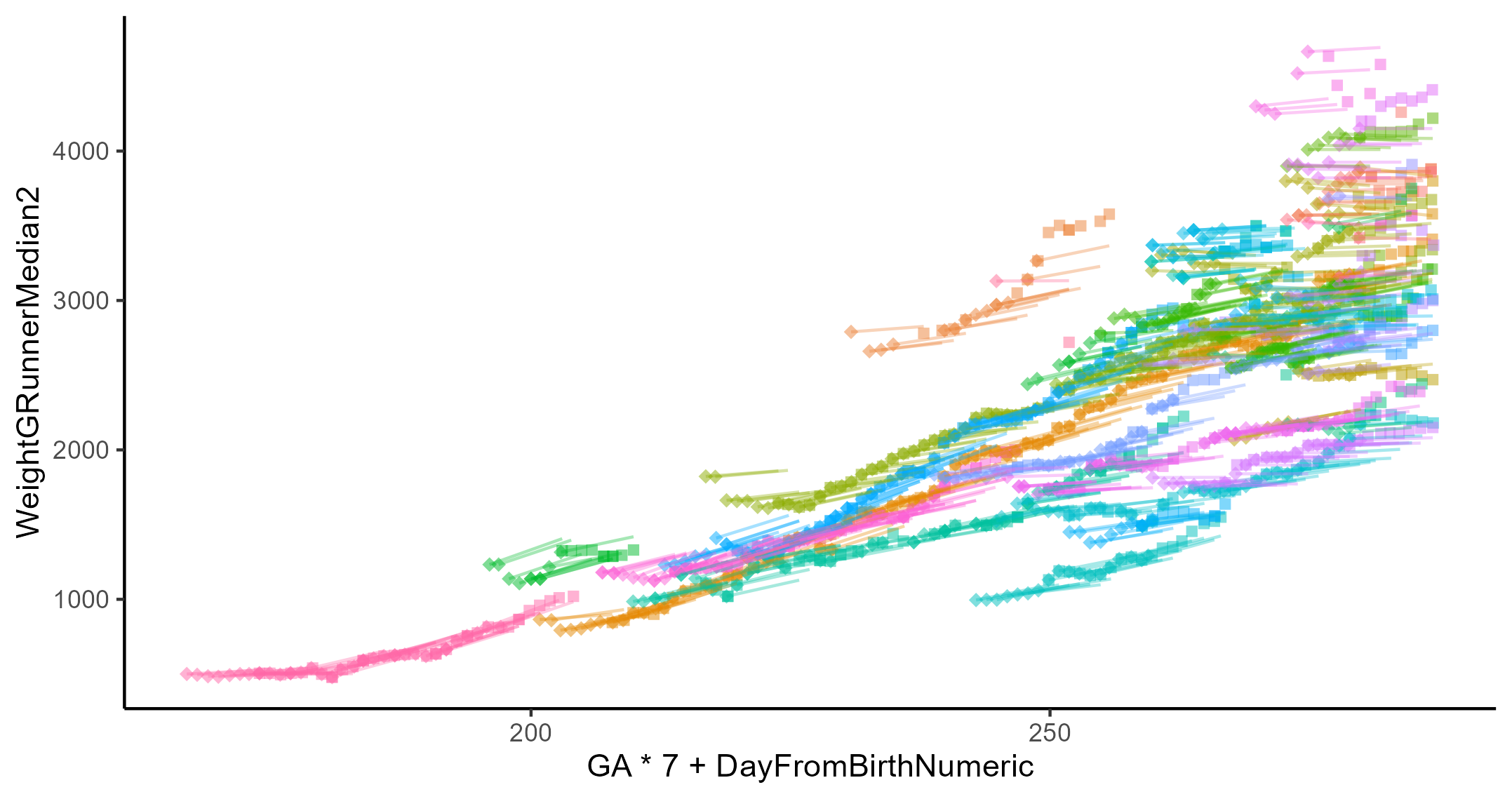 Growth plot of approximately sixty patients. The diamonds and squares represent the beginning and end, respectively, of a 7-day period with recorded weight on both ends. While the line represents modeled growth over the same 7-day span. The x-axis the post menstrual age.
Growth plot of approximately sixty patients. The diamonds and squares represent the beginning and end, respectively, of a 7-day period with recorded weight on both ends. While the line represents modeled growth over the same 7-day span. The x-axis the post menstrual age.
