Neonatal General 12: Prenatal Exposures
Session: Neonatal General 12: Prenatal Exposures
432 - Maternal dosage and urine/cord drug levels of medication for opioid use disorder as predictors of pharmacologic treatment for neonatal opioid withdrawal syndrome
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 432.5799
Nicole M. Connolly, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Jennifer McAllister, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Scott Wexelblatt, Cincinnati Children's Hospital Medical Center, Mason, OH, United States; Cathy Grisby, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Chunyan Liu, Cincinnati Children's Hospital, Cincinnati, OH, United States; Shelley Ehrlich, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
- NC
Nicole M. Connolly, MD (she/her/hers)
Staff Physician
Cincinnati Children's Hospital Medical Center
Cincinnati, Ohio, United States
Presenting Author(s)
Background: Rates of opioid use disorder (OUD) among pregnant women and neonatal opioid withdrawal syndrome (NOWS) remain a major public health issue in the United States. Predictors of which infants are more likely to require pharmacotherapy for NOWS based on objective measures such as dose of medication for opioid use disorder (MOUD) at time of admission, drug levels in maternal urine or infant umbilical cord samples, or presence of polysubstance exposure identified on maternal urine screening could be useful tools in the care of this population.
Objective: To determine if there is an association between MOUD dosage, level of MOUD in maternal urine samples, polysubstance exposure identified on maternal urine, or level of MOUD in infant cord samples and the need for pharmacologic treatment for NOWS.
Design/Methods: We performed a prospective study of maternal-infant dyads recruited from a regional MOUD program from 2020 to 2023. Polysubstance exposure included identification of non-prescribed opioids, cannabis, amphetamines, benzodiazepines, and cocaine. Dyads were included if data was available for maternal MOUD dose, maternal urine samples, and need for NOWS treatment. Unpaired T-test was used to compare infants that did and did not require pharmacologic treatment for NOWS. Receiver operating characteristic (ROC) analysis was performed on all available samples to examine the predictive value of maternal MOUD dose and level in maternal urine and infant cord samples on need for treatment.
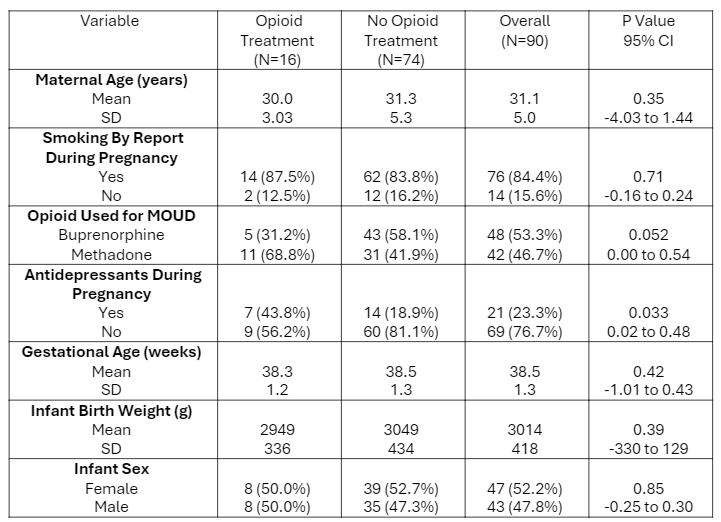
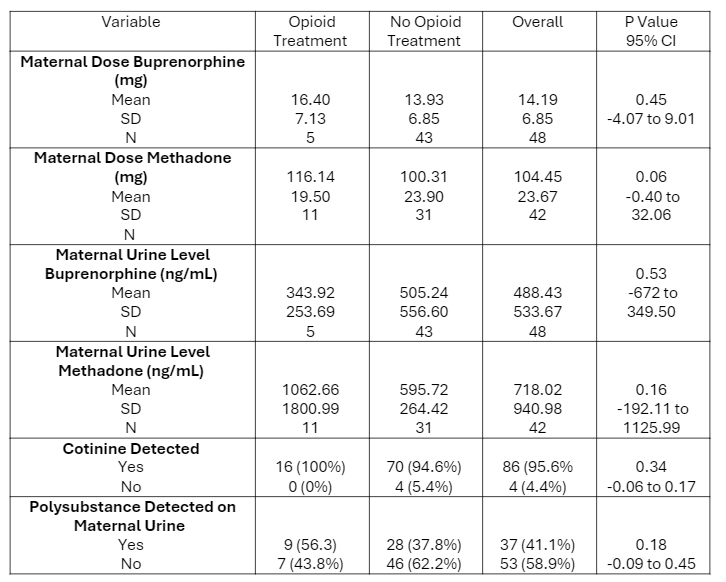
Results: Ninety dyads were identified in the study, of which 16 infants (17.8%) required treatment. No significant differences were found in maternal doses or urine levels of buprenorphine or methadone between infants who required treatment for NOWS and those who did not. Polysubstance exposure did not differ between treated and untreated infants, nor between those exposed to methadone and buprenorphine. Infants exposed to buprenorphine showed a lower likelihood of treatment need though this was not statistically significant (p=0.052, OR 0.33). There was an observed association between maternal antidepressant use and the need for treatment in infants. Cord samples were available for 92 infants, including 10 treated infants, and these levels were not predictive of need for treatment.
Conclusion(s): Maternal MOUD dose and drug levels detected in samples at time of delivery were not associated with the need for pharmacologic treatment for NOWS. Polysubstance exposure identified on maternal urine sample was also not associated with need for treatment. Incidence of NOWS treatment among infants of mothers in this MOUD program remains low.
Demographics
Comparison of MOUD dose, maternal urine levels, and polysubstance exposure in treated and untreated infants
ROC curves and results of ROC analysis predicting opioid treatment by Methadone/Buprenorphine level
.png)

