Neonatal General 11
Session: Neonatal General 11
412 - Sirolimus Use In Neonates With Non-immune Hydrops Fetalis Secondary to Lymphatic Malformations
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 412.6856
Venkatasai P. Devarapalli, UAMS, Little Rock, AR, United States; Makenzie L. Niven, Arkansas Children's Hospital, Little Rock, AR, United States; Prachi Shah, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Andrew Tran, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Vonita Chawla, University of Arkansas for Medical Sciences College of Medicine, Bryant, AR, United States; Shelley E. Crary, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Matthew Merves, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Angela L. Chandler, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Joana Mack, Arkansas Children's Hospital, Little Rock, AR, United States

Venkatasai P. Devarapalli, D.O. (he/him/his)
PGY2
UAMS
Little Rock, Arkansas, United States
Presenting Author(s)
Background: Hydrops fetalis, abnormal fluid accumulation in multiple fetal compartments, is associated with high morbidity and mortality. Recent studies have explored sirolimus, a regulator of lymphangiogenesis and angiogenesis, as a treatment for non-immune hydrops fetalis (NI-HF), particularly in cases involving lymphatic malformations. This approach shows promise for managing large fluid collections that are challenging to address surgically.
Objective: Our study evaluates the clinical impact of sirolimus on neonates with NI-HF secondary to lymphatic malformations.
Design/Methods: We conducted an IRB-approved retrospective, single-institution study of neonates born between 2017 to 2024. Patients diagnosed with NI-HF, central conducting lymphatic anomaly (CCLA), vascular malformation, or lymphatic malformation, were identified using the Epic slicer-dicer tool and clinic lists.
Results: 18 neonatal patients (9 female, 9 male) were included. 11 patients were Caucasian, 3 Hispanic, 1 Black, and 1 unknown. The median gestational age was 32w4d (range 24w3d to 37w0d). Of the 18 patients, 1 was diagnosed with NI-HF prenatally but clinical findings at birth were inconsistent with NI-HF or any lymphatic anomalies. Of the remaining 17 patients, 10 were diagnosed with only NI-HF, 2 had only vascular anomalies (both with cystic lymphatic malformations), and 5 had both NI-HF and vascular anomalies. 7/18 patients had a vascular anomaly and 5/7 received sirolimus. One patient showed spontaneous improvement in clinical condition and did not receive sirolimus and the other passed before sirolimus therapy was initiated due to respiratory failure. The average sirolimus starting dose was 1.048 mg/m2/day and the average dose at which our patients achieved an optimal sirolimus trough was1.43 mg/m2/day.
Out of the 5 patients that received sirolimus, 4 survived. Out of the 12 patients that did not receive sirolimus, 6 survived.
Conclusion(s): Sirolimus demonstrated safety and efficacy in treating NI-HF related to various lymphatic malformations. Patients who responded successfully to sirolimus treatment shared the following key characteristics: prenatal diagnosis, similar starting doses and initiating therapy no earlier than 42 weeks post-menstrual age. No patient stopped sirolimus due to side effects. Our findings are promising and larger prospective studies are needed to validate the use of sirolimus in neonates with NI-HF resulting from underlying vascular and lymphatic anomalies.
Survival Rates of Infants with and without Sirolimus
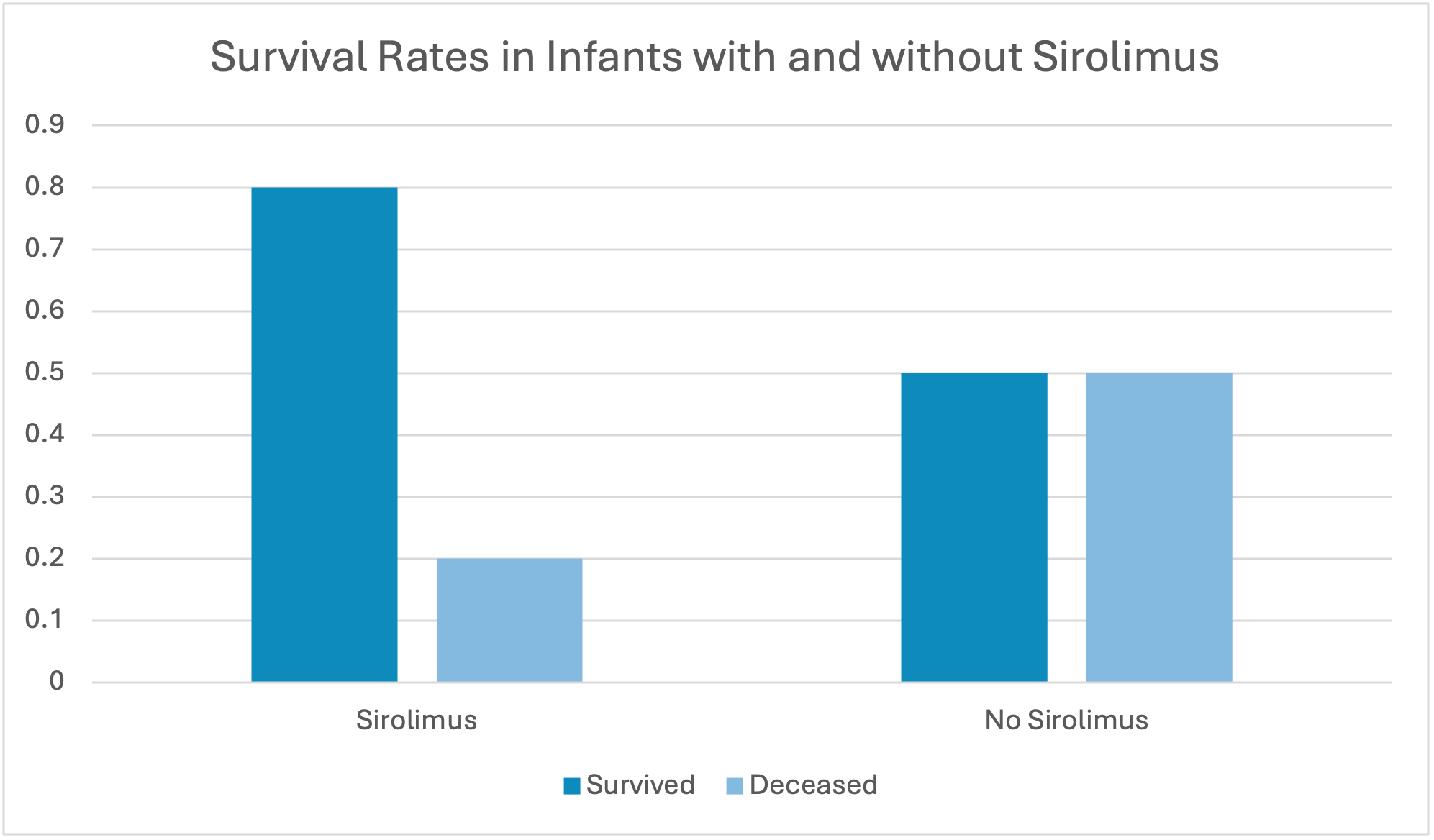 Survival rates of infants on Sirolimus were higher in our study than those not.
Survival rates of infants on Sirolimus were higher in our study than those not. 
