Neonatal Neurology 8: Pre-Clinical 2
Session: Neonatal Neurology 8: Pre-Clinical 2
019 - Combined Sulforaphane and Deferoxamine treatment protects against excessive microglial activation and polarization, and suppresses inflammation and oxidative stress after intraventricular hemorrhage
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 19.4159
Vanessa Castro Diaz, Maria Fareri Children's Hospital at Westchester Medical Center, fort Lee, NY, United States; Michelle A. Sunshine, Maria Fareri Children's Hospital at Westchester Medical Center, Dobbs Ferry, NY, United States; Furong Hu, Boston Children's Hospital, Valhalla, NY, United States; Michael S. Wolin, New York Medical College, Valhalla, NY, United States; Selvakumar Subbian, Rutgers New Jersey Medical School, Newark, NJ, United States; Govindaiah Vinukonda, New York Medical College, Valhalla, NY, United States; Edmund F.. LaGamma, New York Medical College, Valhalla, NY, United States
.jpg)
Vanessa Castro Diaz, MD
Neonatal Perinatal Fellow
Maria Fareri Children's Hospital at Westchester Medical Center
fort Lee, New York, United States
Presenting Author(s)
Background: Intraventricular hemorrhage (IVH) is a common complication of premature birth causing white matter (WM) injury and long-term neuromotor and cognitive disabilities; no definitive treatment exists. Extravasated RBC lysis-released toxic mediators (free hemoglobin [Hb] & Fe2+/3+) trigger an influx of proinflammatory cytokines & generate free radicals. After IVH, resident microglia differentiate into pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes that modulate these responses. The effects of sulforaphane (SFN), a Nrf2 anti-inflammatory transcription factor activator, and deferoxamine (DFN), an iron chelator, are tested after IVH.
Objective: To determine whether combined pharmacological agents SFN-DFN can attenuate excessive microglial activation and associated proinflammatory responses/oxidative stress, which should reduce WM damage after IVH.
Design/Methods: We used our neonatal rabbit model of glycerol-induced IVH (Vinukonda, 2019). After confirming severity of IVH by head ultrasound, rabbit pups (n=5/grp) were treated with 0.9% saline or SFN (25 mg/kg, IM, QOD) + DFN (50 mg/kg, SC, BID) and sacrificed for analysis on day 3 & 7 postnatal age. Immunostaining identified microglial density/polarization; biochemical assays measured free Hb & Fe2+/3+ in cerebrospinal fluid (CSF) & tissue lysates.
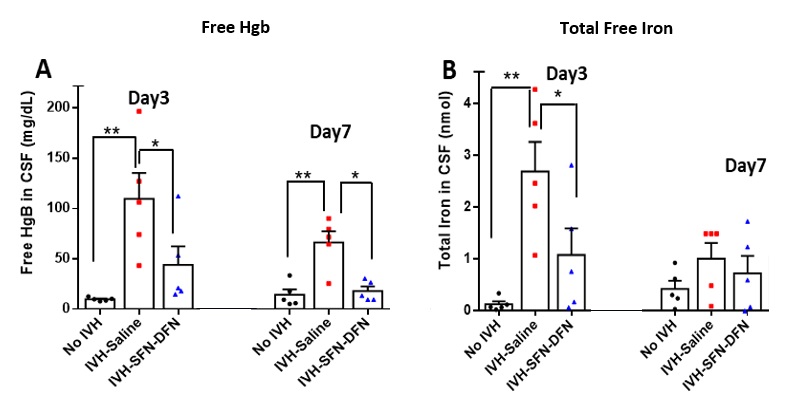
Results: Resident Iba-1+ microglia are densely populated at the borders of the ventricles & choroid plexus. Resident microglia become highly mitotic & polarize into the M1 phenotype (HLA+) increasing fivefold in IVH vs no-IVH pups (p < 0.05, n=5); M2 data is in progress. SFN-DFN treatment reduced M1 microglia by 2.3-fold compared to IVH-saline pups (p < 0.05, n=5) & lowered free Hb & Fe2+/3+ concentrations in CSF by 2.6 & 2.5-fold respectively. Fe2+/3+ concentrations fell by 2.2-fold in tissue lysates (p < 0.05, n=5) along with a 25% increase in superoxide dismutase enzyme levels that suppresses oxidative stress (p < 0.05, n=5). RNA-Seq & TaqMan analysis of the subventricular zone identified that the highest number of dysregulated genes after IVH were related to Fc-R mediated phagocytosis functions of microglia (not shown).
Conclusion(s): SFN-DFN treatment effectively reduced Hb and iron accumulation, mitigated inflammation, and decreased free radicals after IVH. We speculate that a strategy of combined & targeted pharmacological interventions vs monotherapy may be a preferred approach to address this complex injury paradigm at multiple levels, to synergistically reduce WM damage after IVH. Less inflammation may enhance the beneficial effects of stem cell “living therapy” we identified by prolonging cell survival.
Figure 1: Distribution of total microglia (Iba-1+; green) in the developing forebrain in control vs IVH pups (arrows)
.jpg)
Figure 2. CSF concentration of Free Hb (A), and total free iron (Fe2+/3+) (B) on day 3 and 7 postnatal age after IVH-saline vs SFN-DFN treatment.