Neonatal Hematology & Bilirubin Metabolism 2
Session: Neonatal Hematology & Bilirubin Metabolism 2
671 - Comparative Efficacy of 300 u/kg vs. 500 u/kg Dosing of Recombinant Erythropoietin in Preterm Infants
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 671.4143
Eric A. Tano, UH Rainbow Babies & Children's Hospital, Coral Gables, FL, United States; Carolyn Vekstein, Case Western Reserve University School of Medicine, Cleveland, OH, United States; Jaime Marasch, UH Rainbow Babies & Children's Hospital, Cleveland, OH, United States; Rita M.. Ryan, UH Rainbow Babies & Children's Hospital, Cleveland, OH, United States; Mary L. Nock, UH Rainbow Babies & Children's Hospital, Case Western Reserve University School of Medicine, Cleveland, OH, United States

Eric A. Tano, MD
Neonatology Fellow
UH Rainbow Babies & Children's Hospital
Coral Gables, Florida, United States
Presenting Author(s)
Background: Recombinant erythropoietin (EPO) is utilized to prevent and treat anemia of prematurity, aiming to reduce blood transfusions and donor exposures. Previously, EPO was administered at 500 u/kg, 3x/week after 28 days of life. However, this dosing was not well-supported by literature, prompting a protocol change to 300 u/kg 3x/week.
Objective: The primary objective was to evaluate if the reduced EPO dose (300 u/kg) achieves a similar increase in hematocrit (Hct) compared to the previous 500 u/kg dose. Secondary objectives included assessing EPO’s effect on white blood cell count (WBC) and investigating the potential of reticulocyte-hemoglobin (Ret-He) as a marker for iron status during EPO treatment.
Design/Methods: Data for the 500 u/kg cohort had been previously collected, and we retrospectively collected data for infants who received EPO at 300 u/kg following the new protocol. Electronic medical records provided patient demographics, EPO administration details, lab results (before, during, and after EPO), morbidities, and transfusion needs. Paired t-tests were used for continuous, normally distributed variables, and linear regression analyzed continuous variable comparisons.
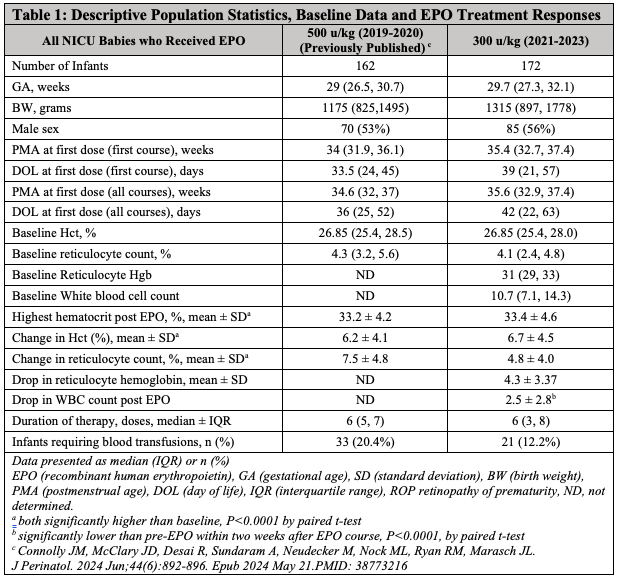
Results: A total of 155 infants received 175 courses of EPO at 300 u/kg, with 18 infants undergoing two courses and two receiving three. Average birthweight was 1315 grams, and mean gestational age was 29.7 weeks. Mean starting Hct was 26.7% (SD 1.8%), and mean reticulocyte count was 4.1% (SD 1.74%). The mean increase in Hct was 6.6% (SD 4.4%), similar to the 500 u/kg dose. However, the 500 u/kg cohort showed a greater reticulocyte count increase (7.5%) than the 300 u/kg cohort (4.9%). WBC significantly declined following EPO administration, with a mean WBC drop of 2.5 (SD 2.8) within two weeks post-EPO (P < 0.0001). Additionally, lower starting Ret-He correlated with a higher Hct increase, and the magnitude of Hct rise was significantly associated with a drop in Ret-He (P=0.011) during the EPO course.
Conclusion(s): Using 300 u/kg EPO dosing resulted in a hematocrit increase comparable to the 500 u/kg dosing, although reticulocyte counts were higher with the 500 u/kg dose, this may be unnecessary given the similar Hct increases. EPO treatment was associated with a statistically significant WBC decrease. Ret-He shows promise as an iron store marker in preterm infants, though further research is needed to clarify its role in managing anemia of prematurity.
Change in Hematocrit and Starting Reticulocyte Hemoglobin
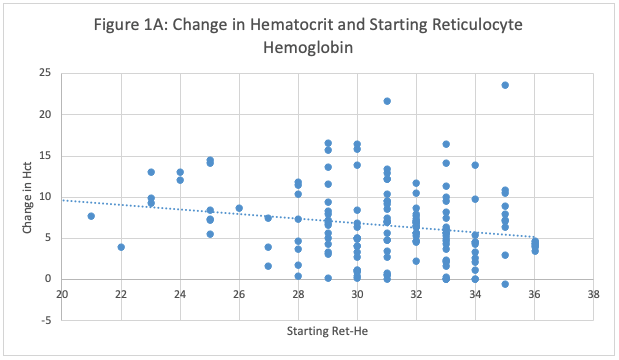 Figure 1A: Relationship Between Baseline Reticulocyte Hemoglobin (Ret-He) and Hematocrit (Hct) Increase in Preterm Infants Treated with 300 u/kg EPO. The figure illustrates the change in hematocrit levels relative to initial reticulocyte hemoglobin values, which serve as an indicator of iron stores. Contrary to expectations, a higher starting reticulocyte hemoglobin level was associated with a lower rise in hematocrit (P <0.0001, by linear regression).
Figure 1A: Relationship Between Baseline Reticulocyte Hemoglobin (Ret-He) and Hematocrit (Hct) Increase in Preterm Infants Treated with 300 u/kg EPO. The figure illustrates the change in hematocrit levels relative to initial reticulocyte hemoglobin values, which serve as an indicator of iron stores. Contrary to expectations, a higher starting reticulocyte hemoglobin level was associated with a lower rise in hematocrit (P <0.0001, by linear regression). Change in Hematocrit and Drop in Reticulocyte Hemoglobin
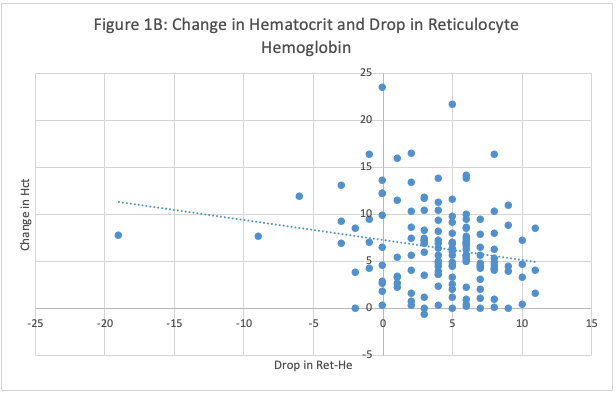 Figure 1B: Association Between Rise in Hematocrit and Drop in Reticulocyte Hemoglobin in Preterm Infants Treated with 300 u/kg EPO. Higher hematocrit responses were associated with a greater decrease in Ret-He (P=0.0101, by linear regression).
Figure 1B: Association Between Rise in Hematocrit and Drop in Reticulocyte Hemoglobin in Preterm Infants Treated with 300 u/kg EPO. Higher hematocrit responses were associated with a greater decrease in Ret-He (P=0.0101, by linear regression). Descriptive Population Statistics, Baseline Data and EPO Treatment Responses
Change in Hematocrit and Starting Reticulocyte Hemoglobin
 Figure 1A: Relationship Between Baseline Reticulocyte Hemoglobin (Ret-He) and Hematocrit (Hct) Increase in Preterm Infants Treated with 300 u/kg EPO. The figure illustrates the change in hematocrit levels relative to initial reticulocyte hemoglobin values, which serve as an indicator of iron stores. Contrary to expectations, a higher starting reticulocyte hemoglobin level was associated with a lower rise in hematocrit (P <0.0001, by linear regression).
Figure 1A: Relationship Between Baseline Reticulocyte Hemoglobin (Ret-He) and Hematocrit (Hct) Increase in Preterm Infants Treated with 300 u/kg EPO. The figure illustrates the change in hematocrit levels relative to initial reticulocyte hemoglobin values, which serve as an indicator of iron stores. Contrary to expectations, a higher starting reticulocyte hemoglobin level was associated with a lower rise in hematocrit (P <0.0001, by linear regression). Change in Hematocrit and Drop in Reticulocyte Hemoglobin
 Figure 1B: Association Between Rise in Hematocrit and Drop in Reticulocyte Hemoglobin in Preterm Infants Treated with 300 u/kg EPO. Higher hematocrit responses were associated with a greater decrease in Ret-He (P=0.0101, by linear regression).
Figure 1B: Association Between Rise in Hematocrit and Drop in Reticulocyte Hemoglobin in Preterm Infants Treated with 300 u/kg EPO. Higher hematocrit responses were associated with a greater decrease in Ret-He (P=0.0101, by linear regression). Descriptive Population Statistics, Baseline Data and EPO Treatment Responses


