Neonatal General 13: Retinopathy of Prematurity
Session: Neonatal General 13: Retinopathy of Prematurity
449 - Effect of Antenatal and Postnatal Steroids in the Development of Retinopathy of Prematurity
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 449.4862
Faiza Khurshid, Queen's University Faculty of Health Sciences, Kingston, ON, Canada; Farnaz Javadian, Queen's University Faculty of Health Sciences, Kingston, ON, Canada; Christine Law, Queen's University Faculty of Health Sciences, Kingston, ON, Canada; Wilma Hopman, Queen's University Faculty of Health Sciences, Kingston, ON, Canada; Brigitte Lemyre, University of Ottawa Faculty of Medicine, Ottawa, ON, Canada; Alana Newman, Dalhousie University Faculty of Medicine, Saint John, NB, Canada; Edith Masse, Université de Sherbrooke, Sherbrooke, PQ, Canada; Jonathan Wong, BC Children’s and Women’s Hospital, Vancouver, BC, Canada; Prakesh S. Shah, Mount Sinai Hospital, Toronto, ON, Canada
- FK
Faiza Khurshid, MD FRCPC (she/her/hers)
Associate Professor
Queen's University Faculty of Health Sciences
Kingston, Ontario, Canada
Presenting Author(s)
Background: Steroid treatment can be crucial in treating morbidity and reducing mortality in preterm infants. The literature describes differing effects of antenatal (ANS) versus postnatal steroids (PCS) in the development of retinopathy of prematurity (ROP).
Objective: In this study, we investigate the relationship between steroid use and the development of ROP in Canadian neonates.
Design/Methods: This is a retrospective cohort study of infants born between January 2010 and December 2022 admitted to level III neonatal intensive care units (NICUs) participating in the Canadian Neonatal Network (CNN) and meeting the inclusion criteria.Data were obtained from the Canadian Neonatal Network (CNN), a national database of all 32 level III neonatal intensive care units (NICUs) in Canada. A total of 20,255 neonates met the inclusion criteria, with admission to a CNN NICU under the gestational age of 30+6 weeks or less than 1250 g, with ROP screening. Unadjusted and adjusted odds ratios based on confounders from conditional logistic regression were determined for the risk of developing any ROP, type 1 ROP, and non-type 1 ROP depending on ANS and PCS use.
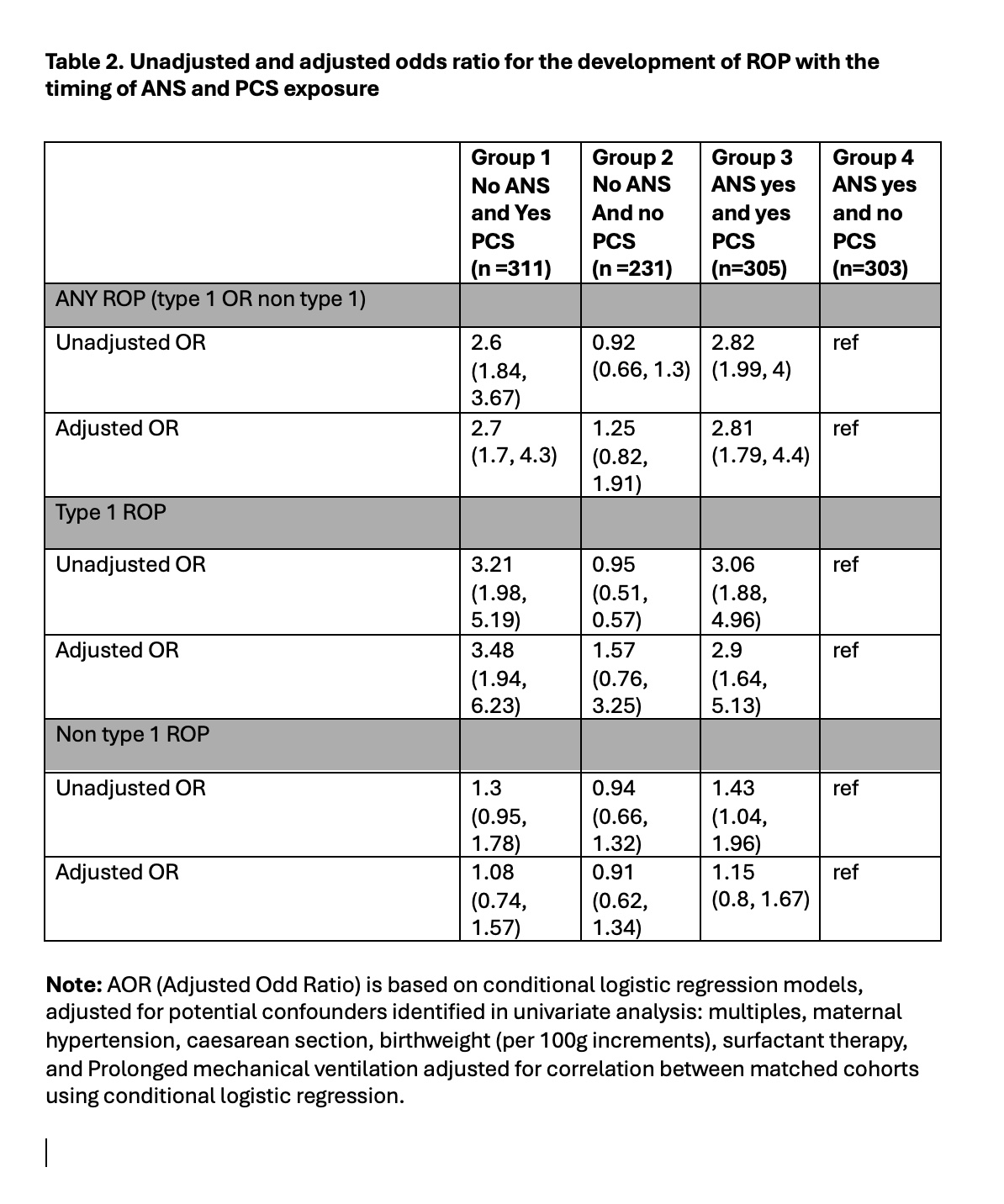
Results: Using ANS only as the reference group, neonates receiving only PCS had the greatest risk of type 1 ROP, with an adjusted odds ratio of 3.48 (1.94, 6.23), followed by neonates receiving both ANS and PCS with an adjusted odds ratio of 2.9 (1.64, 5.13), suggesting a protective effect of ANS. Neonates that received neither ANS nor PCS had an adjusted odds ratio of 1.57 (0.76, 3.25).
Conclusion(s): This is the first large-scale national retrospective review examining the effect of both ANS and PCS on the development of ROP. PCS has a stronger association in the development of type 1 ROP in Canadian neonates. This can be due to a protective effect of ANS, and also a reflection of other morbidity associated with preterm birth such as severe lung disease.
Table 1 Clinical characteristics of study population based on exposure
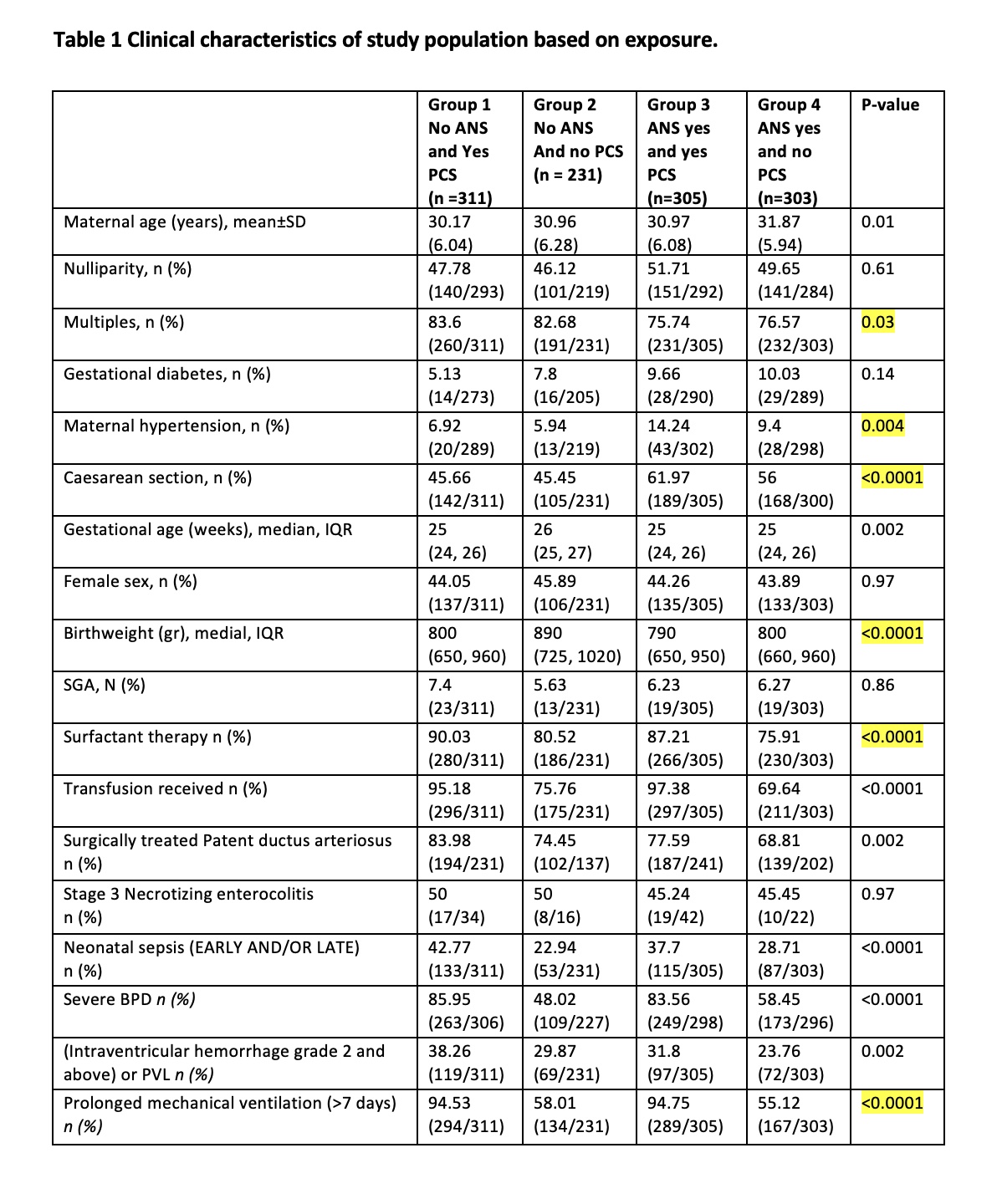
Table 2. Unadjusted and adjusted odds ratio for the development of ROP with the timing of ANS and PCS exposure