Neonatal General
Session: Neonatal General 6: Maternal Fetal Medicine
164 - Benzodiazepine prescriptions during pregnancy, neonatal outcomes, and factors associated with sedative use disorder
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 164.3912
Alexandra Hoeman, USF Health Morsani College of Medicine, Tampa, FL, United States; Tanner Wright, USF Health Morsani College of Medicine, Tampa, FL, United States; Chaitanya Chaphalkar, USF Health Morsani College of Medicine, Tampa, FL, United States; Anthony Kendle, University of South Florida, Tampa, FL, United States
- AH
Alexandra Hoeman, n/a
USF Health Morsani College of Medicine
Tampa, Florida, United States
Presenting Author(s)
Background: Benzodiazepines (BZD) and other sedatives are frequently prescribed during pregnancy despite lacking efficacy for treating mental health disorders and risk of exposure in utero. Sedative exposure may result in neonatal abstinence syndrome (NAS); however, this relationship remains underreported in the current literature.
Objective: As incidence of benzodiazepine use during pregnancy increases, we aimed to describe the association between a prenatal diagnosis of sedative use disorder and delivery of infants with NAS among a cohort of pregnancy-infant dyads with BZD use.
Design/Methods: We conducted a retrospective cohort study of all patients who received prescription BZD during pregnancy and delivered >34 weeks’ gestation at a tertiary care center from January 2014 to June 2024. Patients were excluded if they received BZD only in the context of peri-procedural anesthesia. Medical records were extracted for sociodemographic and neonatal outcomes such as gestational age, anthropometrics, and NICU admission at delivery. International Disease Classification codes identified patients with a diagnosis of sedative use disorder and NAS diagnosis and related symptoms. Groups were compared using chi-square and Fisher’s Exact test. A p< 0.05 was considered significant.
Results: During the study period, 546 deliveries occurred to those prescribed a BZD, with sedative use disorder identified in 23 (4.2%) of deliveries. Those with a diagnosis of sedative use disorder were significantly more likely to deliver an infant diagnosed with NAS compared to those without a diagnosis of sedative use disorder (43.5% vs 8.2%, p = 0.00001). Including infants with an ICD code of “infant affected by maternal use of controlled substance,” but without an NAS diagnosis (P96.1) similarly revealed that infants with symptoms of NAS were significantly more likely to be delivered to those with a sedative use diagnosis than to those without (82.6% vs 31.7%, p = 0.00001). However, there was no significant association between sedative use disorder and NICU admission when compared to those without a diagnosis of sedative use disorder (26.1% vs 19.7%, p = 0.4528).
Conclusion(s): It is important to evaluate for sedative use disorder among patients prescribed BZDs during pregnancy given the prevalence of use in this study. As a diagnosis of sedative use disorder is associated with a diagnosis of NAS in the infant, future research should examine contributors to the diagnostic coding patterns of maternal-infant dyads.
Fig. 3: Sedative use disorder versus no sedative use disorder and NICU admission
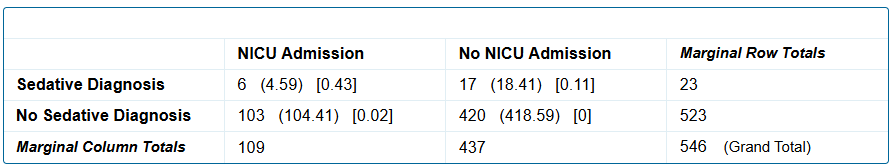 A sedative use disorder diagnosis is not significantly associated with NICU admission status of the infant, p = .4528.
A sedative use disorder diagnosis is not significantly associated with NICU admission status of the infant, p = .4528.Fig. 2: Sedative use disorder versus no sedative use disorder and NAS symptoms
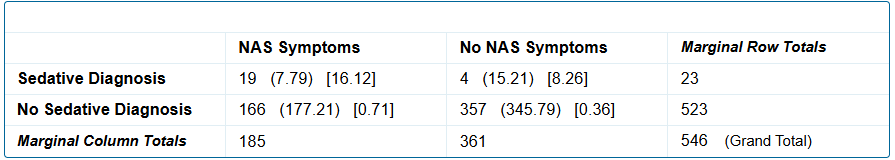 A sedative use disorder diagnosis is significantly associated with the birth of an infant with NAS symptoms, p = 0.00001.
A sedative use disorder diagnosis is significantly associated with the birth of an infant with NAS symptoms, p = 0.00001.Fig.1: Sedative use disorder versus no sedative use disorder and NAS
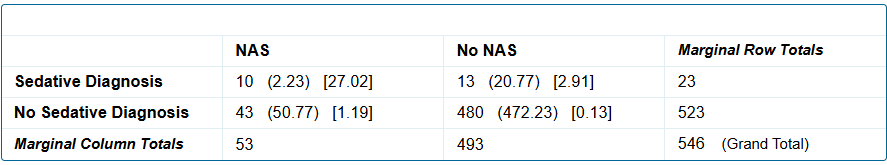 A sedative use disorder diagnosis is significantly associated with the birth of an infant with a diagnosis of NAS, p = 0.00001.
A sedative use disorder diagnosis is significantly associated with the birth of an infant with a diagnosis of NAS, p = 0.00001.
