Genomics/Epigenomics 1
Session: Genomics/Epigenomics 1
382 - Precision Medicine in Bronchiolitis: A Feasibility Study in the Pediatric Emergency Department
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 382.6403
Andrea Rivera-Sepulveda, Nemours Children's Health, ORLANDO, FL, United States; Kenneth A. Alexander, The University of Central Florida, Orlando, FL, United States; Matthew M. Davis, Nemours Children's Hospital, Wilmington, DE, United States; Todd A. Florin, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Kathryn Blake, Nemours Children's Health, Jacksonville, FL, United States; Edward B. Mougey, Nemours Children's Hospital, Jacksinville, FL, United States

Andrea Rivera-Sepulveda, MD, MSc
Associate Professor
Nemours Children's Health
ORLANDO, Florida, United States
Presenting Author(s)
Background: Traditional “one-size-fits-all” bronchiolitis management approaches fail to meet individual children’s needs due to the wide variability in disease response and progression. Precision medicine offers a promising pathway to improve outcomes in bronchiolitis. Implementing DNA-based pharmacogenomic testing in pediatric emergency departments (ED) could offer insights into how genetic variations impact medication responses, potentially reducing unnecessary treatments and enhancing care effectiveness. However, the ED’s fast-paced environment and urgent decision-making present unique challenges for conducting and integrating DNA-based studies.
Objective: To evaluate the feasibility of collecting and processing DNA samples from children with bronchiolitis in the ED.
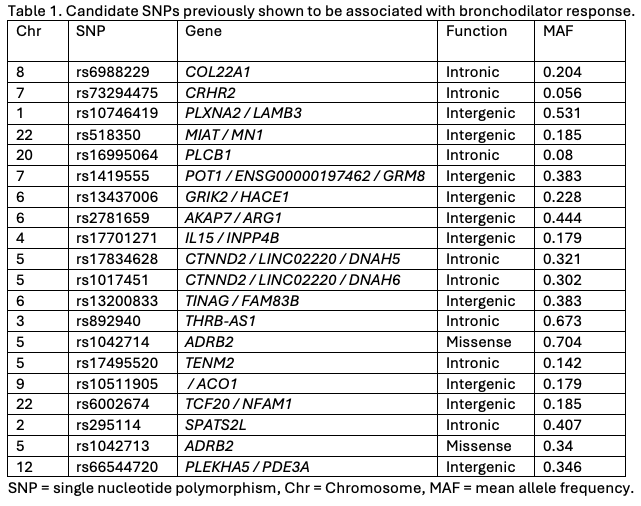
Design/Methods: This feasibility study was embedded within a prospective, double-blind, randomized, placebo-controlled trial of a single albuterol dose in children aged 3 to 24 months presenting to the ED with bronchiolitis. We collected buccal swabs for DNA, measured caregiver consent rates, collection times, and sample processing durations. To identify single nucleotide polymorphisms (SNPs) that predict the therapeutic outcome of bronchodilators for bronchiolitis in infants, candidate SNPs were selected from previously published association studies of pulmonary function outcomes following bronchodilator therapy.
Results: Over 15 months, 82 patients were enrolled, reflecting a 92% caregiver consent rate. All attempts to collect DNA specimens from children were successful (100% collection rate). The mean time for consent to sample collection was 34 minutes, and the collection process was performed in under 5 minutes. Sample transfer and DNA purification were completed in under 45 minutes, aligning with typical ED workflows. All 82 buccal swab specimens passed quality control metrics and were genotyped on the Axiom Precision Medicine Diversity Array. NextGen DNA sequencing turnaround time was 4 weeks.
Conclusion(s): This study demonstrates the feasibility of conducting DNA-based pharmacogenomic research in a pediatric ED setting, highlighting important operational considerations and potential benefits for precision medicine, including predicting therapeutic response and appropriate therapy selection. Streamlined consent and non-invasive collection facilitated this integration, while staff training and timely processing posed challenges. Future studies should explore ways to enhance the integration of pharmacogenomic data to support personalized treatment strategies in emergency care settings.
Candidate single nucleotide polymorphisms previously shown to be associated with bronchodilator response.