Pulmonology
Session: Pulmonology
098 - Analyzing Corticosteroid Prescribing Trends and Therapeutic Outcomes in EVALI Patients
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 98.3891
Abel De Castro, University of Texas Southwestern Medical School, Dallas, TX, United States; Devika R. Rao, University of Texas Southwestern Medical School, Dallas, TX, United States; Stephen A. Hemmerly, University of Texas Southwestern Medical School, Irving, TX, United States

Abel De Castro, MD
Resident
University of Texas Southwestern Internal Medicine Pediatrics Residency
Dallas, Texas, United States
Presenting Author(s)
Background: In 2019, an outbreak of e-cigarette or vaping product use-associated lung injury (EVALI), a severe lung disease caused by e-cigarette use, was identified. Corticosteroids (CS) are commonly used as the mainstay treatment, but the optimal dosing strategy and associated efficacy remains unclear.
Objective: This study aims to determine the clinical characteristics namely symptoms, chest imaging, and inflammatory markers, associated with low versus high dose steroid treatment, and to describe the efficacy of steroid dosing on pulmonary function testing (PFTs).
Design/Methods: A retrospective analysis was performed in patients aged 13-18 years diagnosed with EVALI at Children’s Medical Center Dallas from August 2019 to May 2024. High dose corticosteroids (CS) was defined as ≥ 250 mg/day, and low dose as < 250 mg/day. Data on demographics, clinical presentation, and PFTs were collected. Chi-square analysis was performed for categorical variables (use of oxygen supplementation, symptom category, chest computed tomography (CT) findings) and paired t-testing was used for continuous variables (PFT variables, inflammatory markers) to determine differences between low and high dose CS dosing (GraphPad Prism 10 software, La Jolla, CA). Paired t-testing was used to analyze PFT values pre- and post-CS treatment.
Results: In total, 50 patients were diagnosed with EVALI, 45 of whom were treated with systemic corticosteroids, and had PFTs pre- and post-CS. The high-dose CS group received 500 to 1000 mg of solumedrol per day, while the low-dose group received 24 to 165 mg. Age in the two groups did not differ (average age of 16.4 years) (Table 1). The need for oxygen supplementation (OR =8.29; 95% CI 1.65, 41.58, p = 0.005,) and presence of diffuse ground glass opacities on chest CT (OR = 6.93; 95% CI 1.66, 28.89, p = 0.005) were associated with high-dose. The low-dose group had a lower c-reactive protein (CRP) than the high-dose group (p < 0.001) (Figure 1). The low-dose group had a higher pre-steroid forced vital capacity (FVC) percent predicted (PP) (66.9 ± 18.2 v 87.3 ± 10.9 p = 0.004) and forced expiratory volume in 1 second (FEV1) PP (68.4 ± 16.6 v 92.6 ± 15.1, p = 0.003) (p = 0.004). The high-dose group had an improvement in FVC post-steroids (p = 0.003) (Table 2).
Conclusion(s): Oxygen supplementation and worse chest CT findings were associated with high-dose CS treatment for EVALI. High-dose CS led to improvement in FVC, while low-dose did not. Clinicians can consider high-dose CS for patients with higher inflammatory markers and more severe lung disease, but further prospective studies are needed for confirmation.
Figure 1. Inflammatory markers and pulmonary function testing results in high- and low-dose corticosteroid groups.
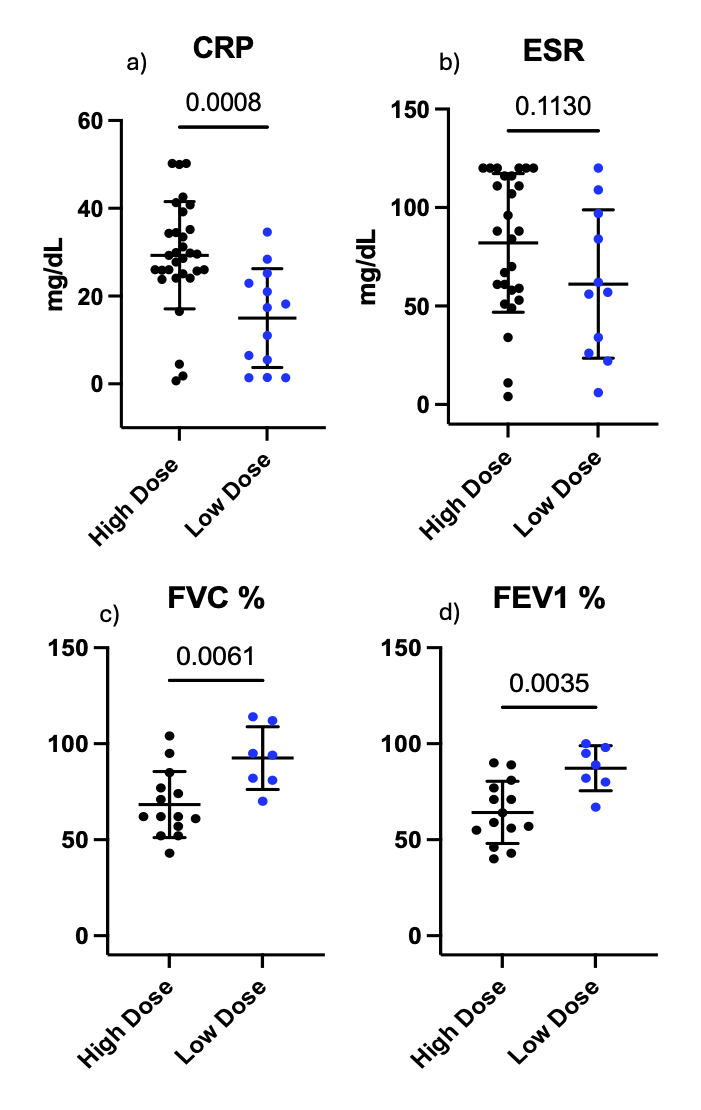 C-Reactive Protein (CRP) (a) for the high-dose group versus the low dose-group (29.3 mg/dL ± 12.2 v 15 mg/dL ± 11.2, p <0.001) while there was no difference between groups with regard to Erythrocyte sedimentation rate (ESR) (b) (71.2 mg/dL ± 32.5 v 61.2 mg/dL ± 37.6, p = 0.113). Both the forced vital capacity % expected (FVC%) (c) (66.9 ± 18.2 v 87.3 ± 10.9 p = 0.004) and forced expiratory volume % expected (FEV1 %) (d) (68.4 ± 16.6 v 92.6 ± 15.1, p = 0.003) were lower in the high-dose group than in the low dose-group.
C-Reactive Protein (CRP) (a) for the high-dose group versus the low dose-group (29.3 mg/dL ± 12.2 v 15 mg/dL ± 11.2, p <0.001) while there was no difference between groups with regard to Erythrocyte sedimentation rate (ESR) (b) (71.2 mg/dL ± 32.5 v 61.2 mg/dL ± 37.6, p = 0.113). Both the forced vital capacity % expected (FVC%) (c) (66.9 ± 18.2 v 87.3 ± 10.9 p = 0.004) and forced expiratory volume % expected (FEV1 %) (d) (68.4 ± 16.6 v 92.6 ± 15.1, p = 0.003) were lower in the high-dose group than in the low dose-group. Table 1. Patient Demographics and Clinical Characteristics
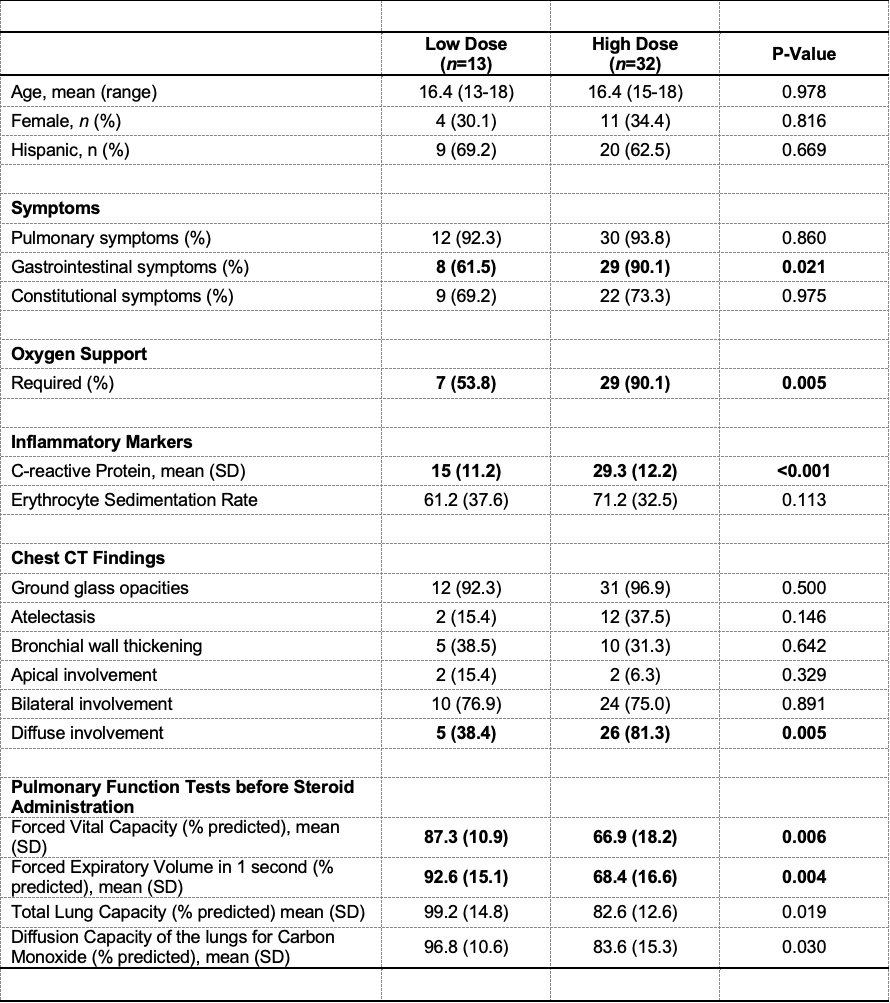 CT = computed tomography
CT = computed tomographyTable 2. Pulmonary Function Testing Results in Low versus High Dosing of Corticosteroids (CS).
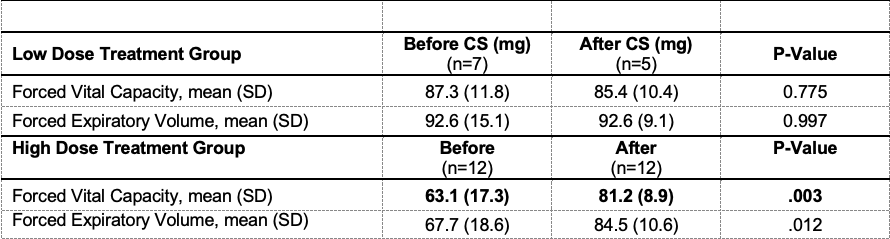 The high dose CS group received between 500 to 1000 mg of solumedrol at the highest point per day while the low dose group received between 24 to 165 mg.
The high dose CS group received between 500 to 1000 mg of solumedrol at the highest point per day while the low dose group received between 24 to 165 mg.
