Neonatal Fetal Nutrition & Metabolism 2
Session: Neonatal Fetal Nutrition & Metabolism 2
630 - Symptomatic Preterm Infants without Aspiration on Swallow Study: Effect of Thickened Liquids
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 630.6923
Katlyn McGrattan, University of Minnesota, Eden Prairie, MN, United States; Gregory P. Jansen, University of Minnesota Masonic Children's Hospital, Minneapolis, MN, United States; Alicia Hofelich mohr, University of Minnesota, Minneapolis, MN, United States; Michael K. Georgieff, University of Minnesota, St. Paul, MN, United States; Sara E. Ramel, University of Minnesota Masonic Children's Hospital, North Oaks, MN, United States; Irena K. Wilson, University of Minnesota, Minneapolis, MN, United States

Katlyn McGrattan, PhD (she/her/hers)
Assistant Professor
University of Minnesota
Eden Prairie, Minnesota, United States
Presenting Author(s)
Background: Approximately 30% of preterm infants require hospitalization at 36 weeks strictly due to oral feeding deficits. A subset of these infants will undergo a videofluoroscopic swallow study (VFSS) to identify the source of these clinical impairments. However, given the VFSS is limited to visualizing just 10% of the infant’s feed, clinicians frequently apply interventions aimed at eliminating aspiration, such as thickening, among symptomatic infants regardless of VFSS results.
Objective: This study was done to elucidate the effect of this practice by examining the effect of thickened liquids on feeding performance among preterm infants who had clinical dysphagia symptoms but did not aspirate on VFSS.
Design/Methods: This retrospective investigation included two cohorts of preterm infants. The first group included infants who did not have a syndrome or genetic anomaly, underwent a VFSS for symptoms of aspiration, did not exhibit aspiration on their exam, but were treated with thickened liquids afterwards. The second control group included age-matched control infants who did not undergo a VFSS and were left on thin liquids throughout their hospital stay (control). Charts of both infants were reviewed for demographics, medical history, and the daily percent of prescribed nutrition consumed by mouth from start of oral feeds until discharge. ANCOVA was used to test for differences in trajectories by thickening and thickening vs control group.
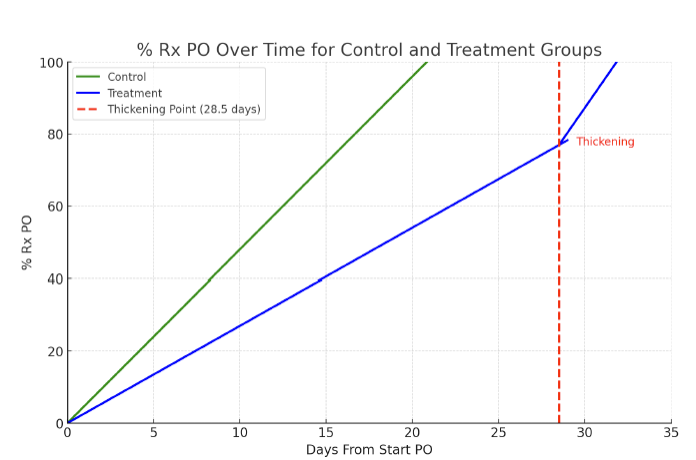
Results: 42 infants were included in the investigation. The thickened group was composed of 21 infants with an average gestational age of 31± 3.5 weeks and postmenstrual age of 42± 3.1 weeks at the start of thickening. The primary comorbidity was BPD (43%), with two infants also having an atrial septal defect. The majority of the thickened infants were placed on mildly thick liquids (86%, N=18), with others placed on slightly (14%, N=3). Prior to the start of thickening, infants in the thickening group exhibited a significantly slower increase in the amount of prescribed oral intake per day than the controls who did not receive thickening (control, 5% increase/day, thickened 3% increase/day)(p <.001). After thickening infants significantly increased their rate of oral intake progression to 7% per day, with average time to full oral intake of 3 days.
Conclusion(s): Limitations in VFSS exams may limit the ability to completely rely on results as a guide for effective feeding interventions. Larger investigations are necessary to determine thickened liquid effects and establish treatment algorithms that consider the risks and benefits.
Percent of Perscribed Oral Intake from Start to Full Oral Feeds in Control and Thickened Groups