Neonatal GI Physiology & NEC 2
Session: Neonatal GI Physiology & NEC 2
721 - Effect of IBP-9414 vs placebo on time to reach sustained full enteral feeding in very low birth weight (VLBW) infants: Results from the “Connection Study”
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 721.4965
Josef Neu, University of Florida College of Medicine, Gaineville, FL, United States; Michael Caplan, Endeavor Health, evanston, IL, United States; Teresa Del Moral, UNIVERSITY OF MIAMI, Miami, FL, United States; Scott Guthrie, Vanderbilt University School of Medicine, Jackson, TN, United States; Mark L. Hudak, University of Florida College of Medicine - Jacksonville, Jacksonville, FL, United States; Jae H. Kim, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Anders Kronström, Infant Bacterial Therapeutics, Stockholm, Stockholms Lan, Sweden; Camilia R. Martin, Weill Cornell Medicine, New York, NY, United States; Neena Modi, Imperial College London, London, England, United Kingdom; Jonas Rastad, Infant Bacterial Therapeutics Inc, Stockholm, Stockholms Lan, Sweden; Rachana Singh, Tufts University School of Medicine, Boston, MA, United States; Staffan Strömberg, Infant Bacterial Therapeutics, STOCKHOLM, Stockholms Lan, Sweden; Hania Szajewska, The Medical Univ of Warsaw, Warsaw, Mazowieckie, Poland; Marcus Thuresson, IBT, Uppsala, Uppsala Lan, Sweden; Flavia Indrio, UNIVERSITY OF SALENTO LECCE ITALY, LECCE, Puglia, Italy

Josef Neu, Doctor of Medicine (he/him/his)
Professor
University of Florida College of Medicine
Gaineville, Florida, United States
Presenting Author(s)
Background: Achievement of full enteral feedings is an important milestone for VLBW preterm infants. We report results of the ‘Connection Study’, a prospective, randomized phase 3 trial on the efficacy and safety of IBP-9414 (NCT03978000) conducted under US IND and EU Clinical Trial Exemptions in 95 neonatal intensive care units.
Objective: Measure effect of IBP-9414 on time to reach sustained full enteral feeding in VLBW infants
Design/Methods: 2117 VLBW infants born at a gestational age between 23 and 32 weeks were randomized to 1:1 treatment with IBP-9414 or placebo. Infants received an initial dose of study drug within 48 hours after birth and then one dose per day until a postmenstrual age of 346/7 weeks. A primary study outcome was the time to sustained feeding tolerance (SFT) defined as a 10-day consecutive time during which nutrition was exclusively enteral (feedings >= 120 ml/kg/d) and average weight gain of at least 10g/kg/day. An alternate time period of 5 consecutive days was analyzed post hoc. Advancements in feeding were determined by individual NICU practice. Analysis of the time to SFT was stratified for birth weight and adjusted for competing risk.
Results: Median birth weight, gestational age at birth, and length of treatment were 850 g, 27 weeks, and 50 days, respectively. SFT was reached by 75% of IBP-9414 treated infants and 71% of those receiving placebo and the median time to SFT was not statistically different but numerically shorter (21 days vs. 23 days) in the IBP-9414 group (p=0.07, Fig. 1). Subgroup analysis demonstrated a statistically significant difference in SFT for infants with a birth weight of 500-749 g (p=0.04) and those born in the United States (p=0.03). Feeding patterns varied widely among the 95 study sites (Fig. 2). A sensitivity analysis using a less stringent 5-day consecutive time endpoint demonstrated an increase in the proportion of infants reaching SFT (83% vs. 77%) and a reduced time to SFT (18 days vs. 20 days, p=0.02). There were no safety concerns as the incidence of adverse events and serious adverse events. There were similar with no cases of IBP-9414 bacteremia.
Conclusion(s): Treatment with IBP-9414 resulted in a non-significant reduction of time to SFT. The unexpected large variability in feeding patterns across study sites may have obscured a treatment effect. Additional analyses demonstrated significant improvement in time to SFT among infants treated with IBP-9414 in important sub-populations. We observed no safety concerns associated with IBP-9414 treatment. These results support a positive benefit:risk profile for IBP-9414 treatment for enteral feeding.
Fig. 1. Forest plot of proportions of infants reaching SFT and the time to SFT in the overall population as well as subgroups thereof
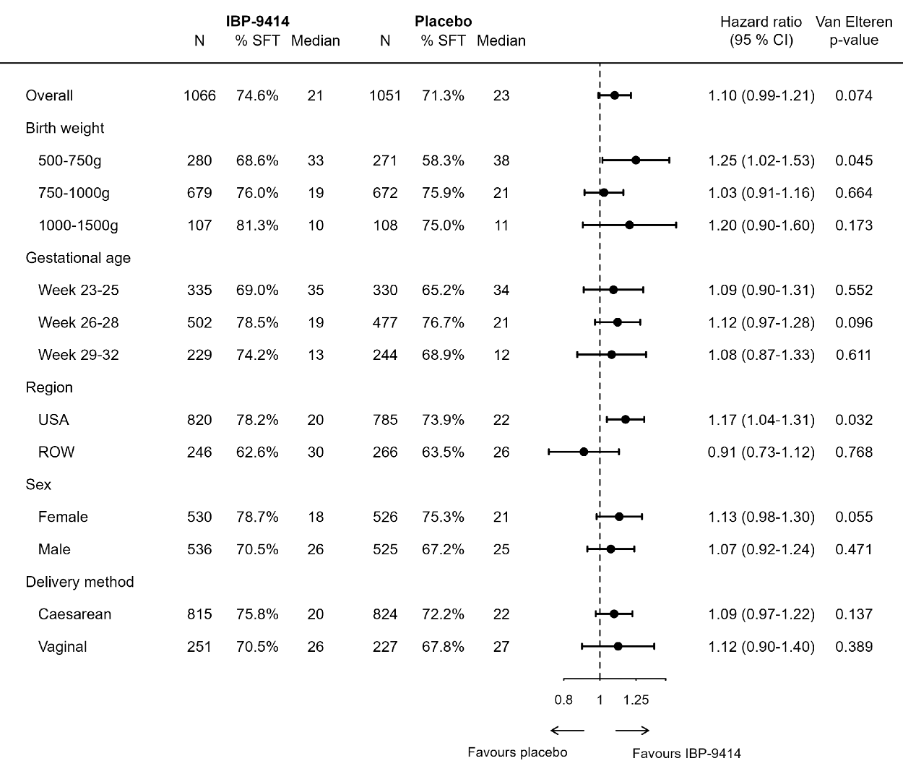
Fig. 2.
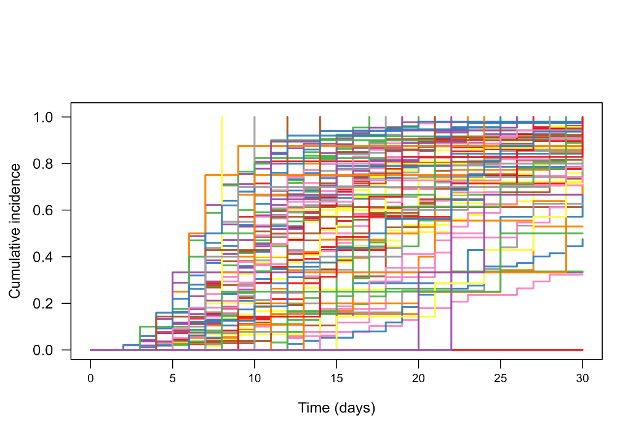 Fig. 2. Median volumes of total, daily enteral feed over time at each of the 95 intensive care units.
Fig. 2. Median volumes of total, daily enteral feed over time at each of the 95 intensive care units.
