Children with Chronic Conditions 1
Session: Children with Chronic Conditions 1
491 - An Enhanced Home-Based Telemedicine Program Using Remote Examination Devices for Children with Medical Complexity: A Pragmatic Randomized Control Trial (RCT)
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 491.3859
Ricardo A.. Mosquera, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Jessica O’Neill, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Julie Eapen, Children's Memorial Hermann Hospital, houston, TX, United States; Maria Caldas-Vasquez, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Tomika S. Harris, UTHealth Houston, Houston, TX, United States; Teddy Gonzalez, University of Texas Medical Branch School of Medicine, Richmond, TX, United States; Maria E. Tellez, McGovern Medical School at the University of Texas Health Science Center at Houston, Pearland, TX, United States; Mar Romero-Lopez, University of Texas Health Science at Houston, Houston, TX, United States; Ara F. Luz, UTHealth Houston, Houston, TX, United States; Matthew Hall, Children's Hospital Association, Lenexa, KS, United States; Sreejata Dutta, Children's Hospital Association, Lenexa, KS, United States; Jay Berry, Complex Care, Boston Children's Hospital, Boston, MA, United States; Giuseppe N.. Colasurdo, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Aravind Yadav, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States
- RM
Ricardo A. Mosquera, MD MS
Professor of Pediatrics
McGovern Medical School at the University of Texas Health Science Center at Houston
Houston, Texas, United States
Presenting Author(s)
Background: Optimizing telemedicine (TM) is essential to reduce infection risk and adverse outcomes in children with medical complexity (CMC), who represent 40-50% of pediatric deaths and hospital costs. Our previous RCT indicated that a conventional telemedicine program, combining comprehensive care (CC) and video/audio likely decreased serious illnesses and days of care away from home.
Objective: We proposed a randomized study in CMC to evaluate whether an enhanced telemedicine program (ETM) using mobile devices for heart and lung auscultation and throat and ear examination can further improve outcomes.
Design/Methods: A single-center randomized clinical trial comparing EMT+CC relative to regular TM+CC for CMC in reducing care days outside the home (clinic, emergency department, or hospital; primary outcome), rate of children developing serious illnesses (death, ICU admission, or hospital stay>7 days). We used Bayesian analyses with neutral prior assuming no benefit. All participants received comprehensive care with 24/7 phone access to primary care providers (PCPs), low patient-to-PCP ratio, and regular TM. The ETM group received remote exam devices, TytoCare, and were instructed to use the devices and to send physical exam results to the clinic via email when patients were sick.
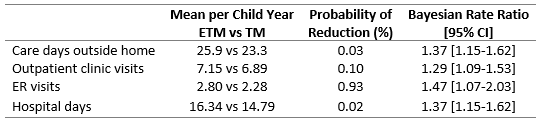
Results: From August 2021 to August 2024, we randomly assigned 254 CMC (130 with ETM vs 124 with TM). Median age was 5.7 years (IQR:3.2,9.5) and 32.7% had positive pressure ventilation at baseline. Median follow-up was 2.1 years (IQR:1.6,2.2) during which children experienced a median of 1 ER visit (IQR: 0-4), 13 inpatient days (IQR: 7-21), and 6 outpatient clinic visits (IQR:4, 9) for a total of 21 care days outside the home (IQR:12,34). On average, patients in the ETM group participated in 1 ETM per patient during the study period. Only 38% of the patients in the ETM group used the device. The probability of a reduction with CC with ETM vs CC with TM was 0.03% for care days outside the home (25.9 vs 23.3 per child-year; Bayesian rate ratio, 1.37 [95% credible interval,1.15-1.62]) and 9.45% for the rate of serious illness (1.07 vs 0.82 per child-year; rate ratio, 1.33 [0.87–2.02]).
Conclusion(s): ETM with CC did not reduce care days outside the home. Under the conditions of the study, the devices were not utilized for this largely Medicaid population of medically complex children. Families were reluctant to adopt the new technology due to longer visit durations, technical challenges, and issues with patient cooperation. Despite the innovation of ETM, they preferred the efficiency of standard TM visits, which lack these additional burdens.
Results of Bayesian Negative Binomial Regression Analysis