General Pediatrics 4
Session: General Pediatrics 4
232 - Clinician preferences for oseltamivir treatment of children with influenza in the outpatient setting
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 232.4250
Hannah Bassett, Stanford University School of Medicine, Redwood City, CA, United States; Suchitra Rao, University of Colorado School of Medicine, Aurora, CO, United States; Jimmy B. Beck, Seattle Children's Hospital, Seattle, WA, United States; Patrick W. Brady, Cincinnati Children's Hospital, Cincinnati, OH, United States; Ravi Jhaveri, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Torsten Joerger, Children's Hospital of Philadelphia, Philadelphia, PA, United States; Danni Liang, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Ricardo Quinonez, Baylor College of Medicine, Houston, TX, United States; Alaina M. Shine, Seattle Children's, Seattle, WA, United States; Maya E. Somers, Lucile Packard Children's Hospital Stanford, San Francisco, CA, United States; Brian P. Lucas, The Dartmouth Institute of Health Policy & Clinical Practice, Norwich, VT, United States; Alan Schroeder, Stanford University School of Medicine, Palo Alto, CA, United States

Hannah K. Bassett, MD
Clinical Associate Professor
Stanford University School of Medicine
Redwood City, California, United States
Presenting Author(s)
Background: Influenza is a common cause of disease in children, with almost 10% of children in the US infected every year. Clinical trials have largely shown a modest reduction in duration of symptoms when the antiviral oseltamivir is started within the first 48 hours of illness. Professional societies recommend antiviral treatment for all children who present within 48 hours of symptoms or for any high-risk children, including children under 2 years and those with comorbid conditions, regardless of illness duration. However, prior evidence has shown not all children receive oseltamivir in accordance with recommendations.
Objective: Our objective was to assess pediatric clinician preferences for oseltamivir use in children with influenza in the outpatient setting.
Design/Methods: We administered a vignette-based survey at 7 university-affiliated children’s hospitals and associated community sites. Attendings, fellows, and advanced practice providers in general pediatrics (GP), emergency medicine (PEM), and infectious disease (PID) were recruited. Our primary outcome was the likelihood to recommend oseltamivir in 4 clinical vignettes, 1 of which was randomized (50:50) to have different illness duration. Likelihood was measured on a 4-point Likert scale from extremely likely to extremely unlikely and assessed independently for each vignette and as a combined outcome across all vignettes. Wave analysis was conducted to assess for non-response bias.
Results: Of 1124 eligible participants, 425 (37.8%) completed surveys. Wave analysis demonstrated no association between survey response time and likelihood to recommend oseltamivir. Participants were somewhat or extremely likely to recommend oseltamivir in 36.2% of cases, however there was variability by specialty from 29.6% to 48.6% (p < 0.001), and study site from 28.6% to 50.7% (p < 0.001). Longer duration of illness (2 vs 4 days) significantly decreased respondents’ likelihood to recommend oseltamivir from 30.1% to 1.9% (p < 0.0001). Overall, we found a discordance rate of 51.8% with national treatment recommendations.
Conclusion(s): We demonstrated considerable non-adherence to national influenza treatment recommendations and variability around use of oseltamivir to treat children with influenza in the outpatient setting. This indicates ongoing uncertainty of the benefit of using oseltamivir in a relatively healthy pediatric population with non-severe disease. Further work is needed to understand factors driving provider preferences. Randomized trials of oseltamivir in previously defined high-risk outpatient pediatric groups would help inform treatment decisions.
Table 1. Characteristics of survey respondents
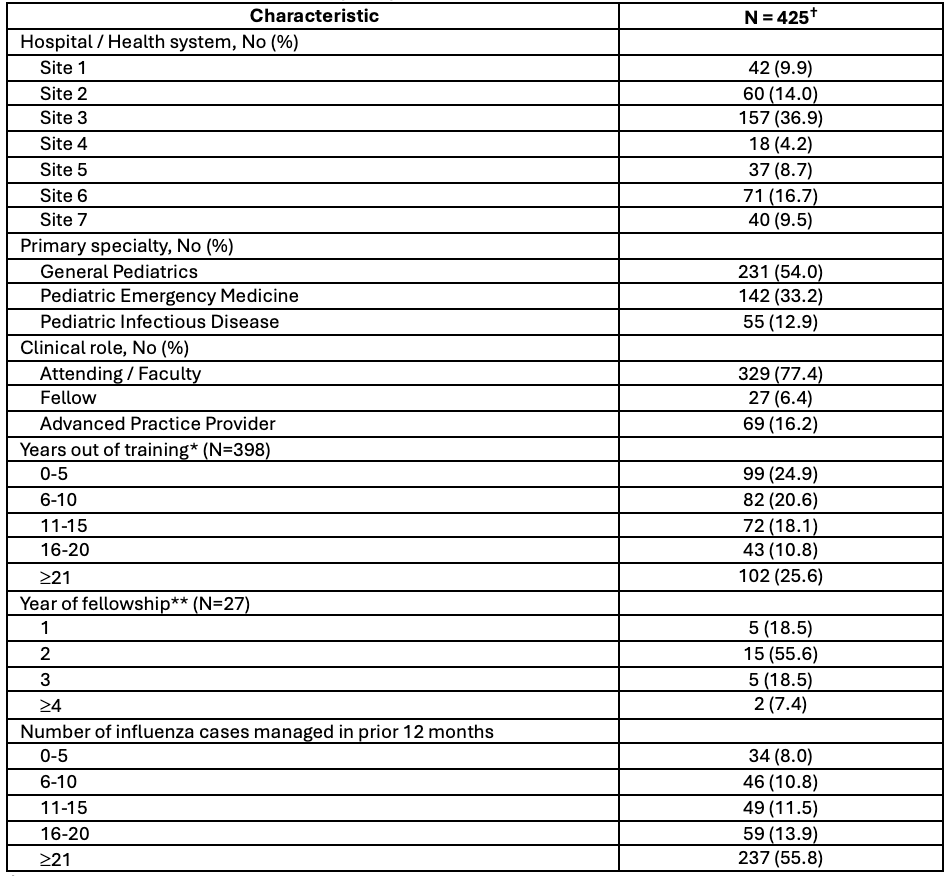 †Numbers for demographic groups may not add up to 425 as not all respondents completed all questions of the survey
†Numbers for demographic groups may not add up to 425 as not all respondents completed all questions of the survey*For attendings / faculty and advanced practice providers
**For respondents who were actively enrolled in pediatric fellowship programs
Table 2. Survey vignettes
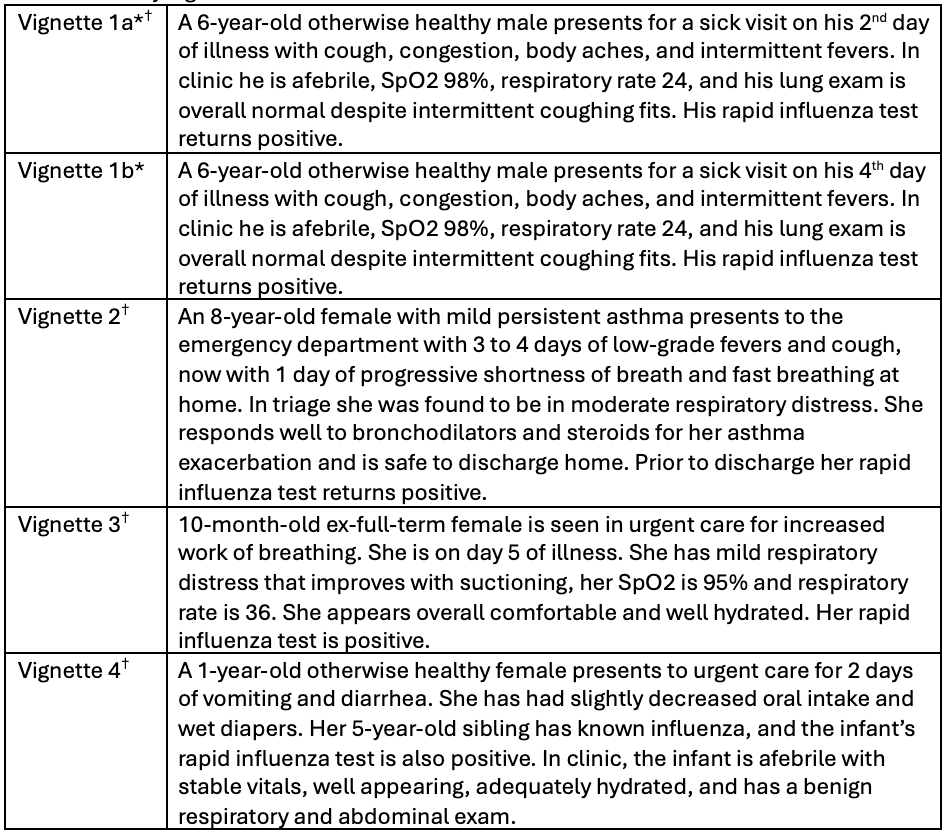 *Vignette 1 was randomized amongst respondents 50:50 into 1a and 1b.
*Vignette 1 was randomized amongst respondents 50:50 into 1a and 1b.†These vignettes represent patients who would warrant treatment with oseltamivir per national recommendations
Figure 1
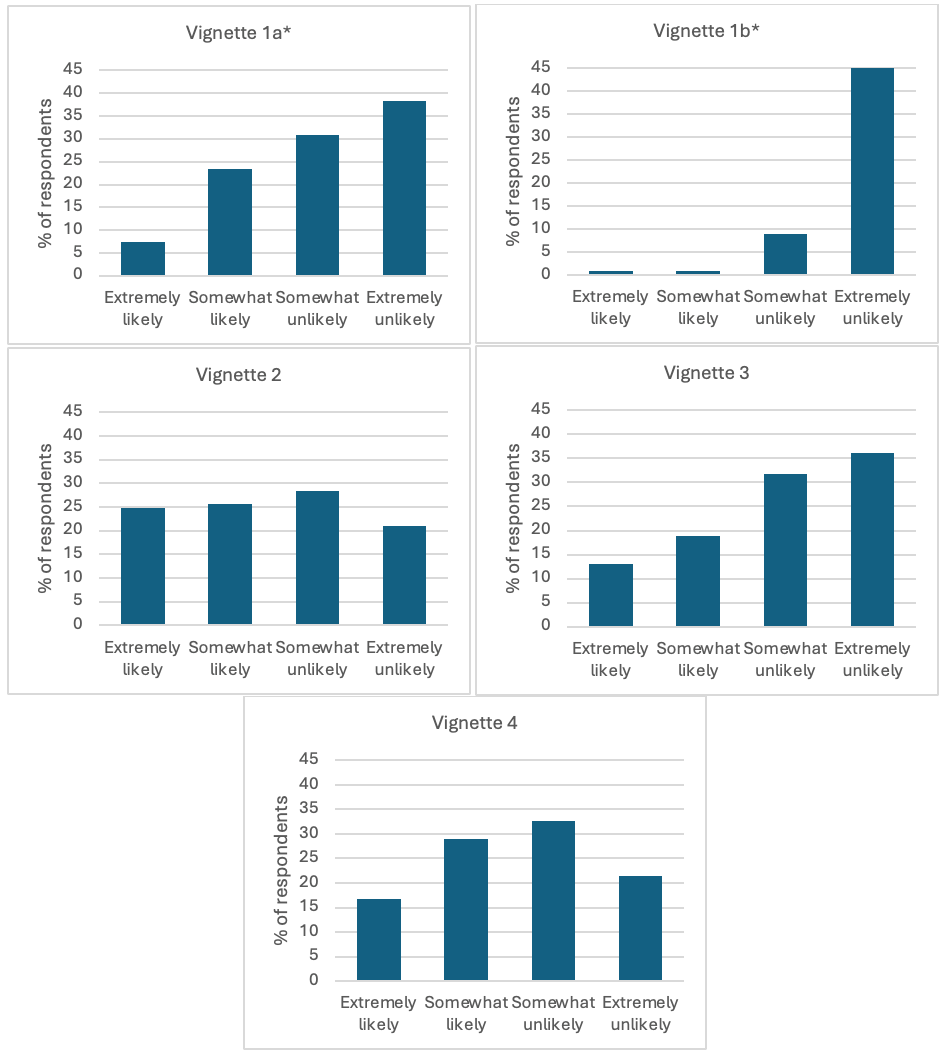 Unadjusted likelihood to recommend oseltamivir for each clinical vignette. *Significant difference (p < 0.001) in likelihood to recommend oseltamivir between the randomized version of vignette 1 (a vs b).
Unadjusted likelihood to recommend oseltamivir for each clinical vignette. *Significant difference (p < 0.001) in likelihood to recommend oseltamivir between the randomized version of vignette 1 (a vs b).
