Global Neonatal & Children's Health 3
Session: Global Neonatal & Children's Health 3
760 - Maternal Cytomegalovirus Seroprevalence in Three Urban Areas of The Americas
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 760.5797
Alvaro Proaño, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Monica Jehnny. Pajuelo, Universidad Peruana Cayetano Heredia, Lima, Lima, Peru; Sylvia Becker-Dreps, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, United States; Filemón Bucardo Bucardo, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, United States; Alvaro Zevallos Barboza, Childrens Hospital of Philadelphia, Arlington, VA, United States; Ravit Arav-Boger, Medical College of Wisconsin, Milwaukee, WI, United States; Robert Gilman, Johns Hopkins University School of Medicine, Baltimore ,, MD, United States; Natalie M. Bowman, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, United States; Dustin D. Flannery, Childrens Hospital of Philadelphia, Philadelphia, PA, United States

Alvaro Proaño, MD
Neonatal-Perinatal Medicine Fellow
Childrens Hospital of Philadelphia
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: Higher maternal cytomegalovirus (CMV) seroprevalence is associated with higher congenital CMV rates worldwide. In the Americas, there is a paucity of published data regarding maternal CMV seroprevalence, especially from lower- and middle-income countries (LMICs), with published data from the 1980s in Peru and no prior data from Nicaragua.
Objective: To determine seroprevalence of maternal CMV from three urban areas in the Americas: one from South America, Central America, and North America.
Design/Methods: Secondary retrospective observational study that leveraged remnant specimens and data from three maternal cohorts: Lima, Peru (2007-2011); León, Nicaragua (2017-2018); and Philadelphia, USA (2020-2023). Sera were tested using the CMV immunoglobulin G ELISA from Zeus Scientific, using CMV strain AD169. An optic density ≤ 0.90 was deemed seronegative, between 0.91 and 1.09 equivocal, and ≥ 1.10 seropositive. Descriptive statistics were used to report demographics of each maternal cohort. Seroprevalence for each cohort was calculated as number of seropositive samples divided by the total number of samples, with 95% confidence intervals (Wald method). Characteristics between CMV seropositive and seronegative patients were compared using standard descriptive statistics. R version 4.3.1 was used for analysis.
Results: A total of 1,076 maternal serum samples were tested; from Peru (N=180), Nicaragua (N=396) and the USA (N=500). CMV seroprevalence was 99.4% (95% CI, 98.4%–100%)in Peru, 99.2% (95% CI, 98.4%–100%), in Nicaragua, and 64.4% (95% CI, 60.2%–68.6%) in the USA . Demographics for each cohort are shown in Table 1. Compared to the Peruvian and Nicaraguan cohorts, the USA cohort was older and utilized more private insurance. In the USA cohort, mothers who were seropositive for CMV were younger, had higher gestation/parity, were more often obese, had higher rates of Black race and Hispanic ethnicity, and higher rates of public insurance (Table 2).
Conclusion(s): In this multi-country maternal seroepidemiology study, we found that CMV seroprevalence was nearly universal among the Peru and Nicaragua cohorts and was substantially higher compared to the USA. This may be due to early exposure to CMV in these populations. Additional research is warranted in understanding the at what age is seroconversion occurring in these populations. In the USA cohort, several risk factors for seropositivity were identified. Further research is needed to understand congenital CMV transmission in LMICs, where maternal CMV seroprevalence is high, as either a re-activation or re-infection of CMV during pregnancy.
Table 1 – Demographics for each Cohort
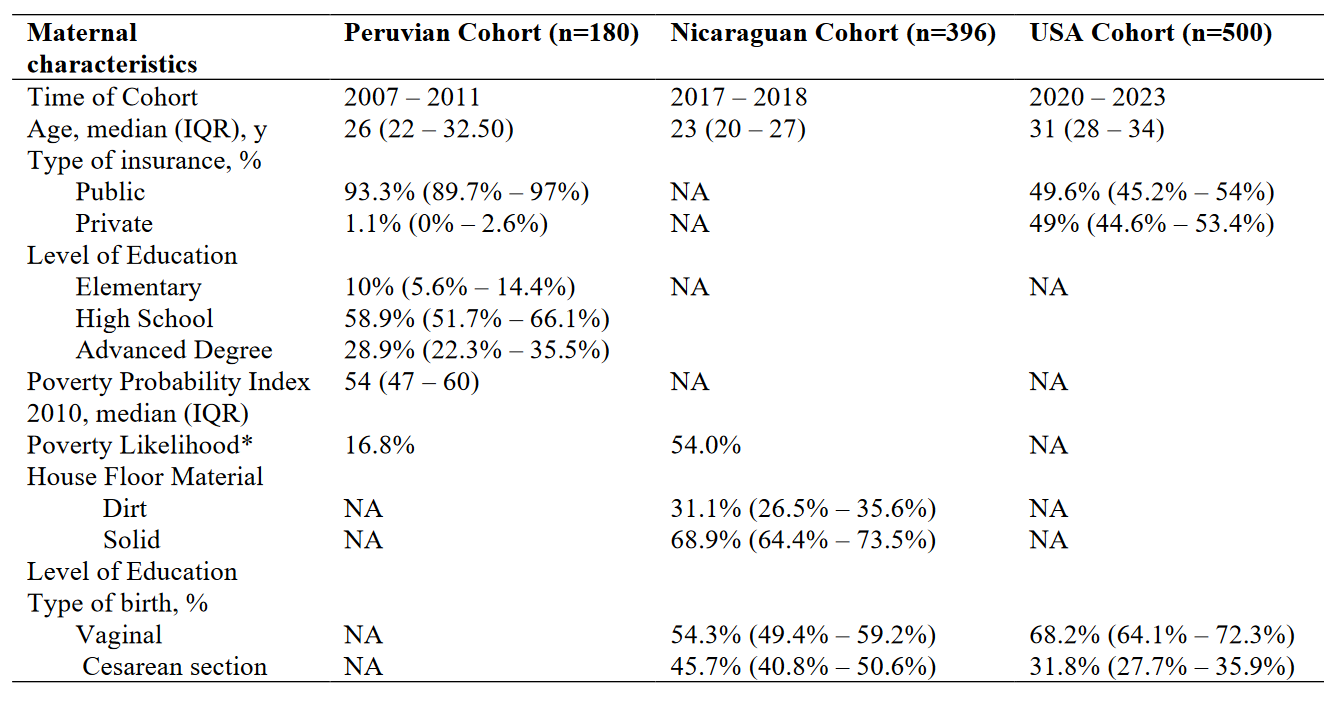 *Poverty likelihood was calculated differently for Peruvian and Nicaraguan Cohorts, the Peruvian Cohort utilized the Poverty Probability Index published in Ballard SB, Requena D, Mayta H, Sanchez GJ, Oyola-Lozada MG, Colquechagua Aliaga FD, Cabrera L, Vittet Mondonedo MD, Taquiri C, Tilley CDH, Simons CMP, Meza RA, Bern C, Saito M, Figueroa-Quintanilla DA, Gilman RH. Enteropathogen Changes After Rotavirus Vaccine Scale-up. Pediatrics. 2022 Jan 1;149(1):e2020049884. doi: 10.1542/peds.2020-049884. PMID: 34918158; PMCID: PMC9647525, while the Nicaraguan Cohort used the Poverty Index published in Vielot NA, González F, Reyes Y, Zepeda O, Blette B, Paniagua M, Toval-Ruíz C, Diez-Valcarce M, Hudgens MG, Gutiérrez L, Blandón P, Herrera R, Cuadra EC, Bowman N, Vilchez S, Vinjé J, Becker-Dreps S, Bucardo F. Risk Factors and Clinical Profile of Sapovirus-associated Acute Gastroenteritis in Early Childhood: A Nicaraguan Birth Cohort Study. Pediatr Infect Dis J. 2021 Mar 1;40(3):220-226. doi: 10.1097/INF.0000000000003015. PMID: 33464013; PMCID: PMC7878336.
*Poverty likelihood was calculated differently for Peruvian and Nicaraguan Cohorts, the Peruvian Cohort utilized the Poverty Probability Index published in Ballard SB, Requena D, Mayta H, Sanchez GJ, Oyola-Lozada MG, Colquechagua Aliaga FD, Cabrera L, Vittet Mondonedo MD, Taquiri C, Tilley CDH, Simons CMP, Meza RA, Bern C, Saito M, Figueroa-Quintanilla DA, Gilman RH. Enteropathogen Changes After Rotavirus Vaccine Scale-up. Pediatrics. 2022 Jan 1;149(1):e2020049884. doi: 10.1542/peds.2020-049884. PMID: 34918158; PMCID: PMC9647525, while the Nicaraguan Cohort used the Poverty Index published in Vielot NA, González F, Reyes Y, Zepeda O, Blette B, Paniagua M, Toval-Ruíz C, Diez-Valcarce M, Hudgens MG, Gutiérrez L, Blandón P, Herrera R, Cuadra EC, Bowman N, Vilchez S, Vinjé J, Becker-Dreps S, Bucardo F. Risk Factors and Clinical Profile of Sapovirus-associated Acute Gastroenteritis in Early Childhood: A Nicaraguan Birth Cohort Study. Pediatr Infect Dis J. 2021 Mar 1;40(3):220-226. doi: 10.1097/INF.0000000000003015. PMID: 33464013; PMCID: PMC7878336. Table 2 – Risk Factors for CMV Positivity in the USA Cohort
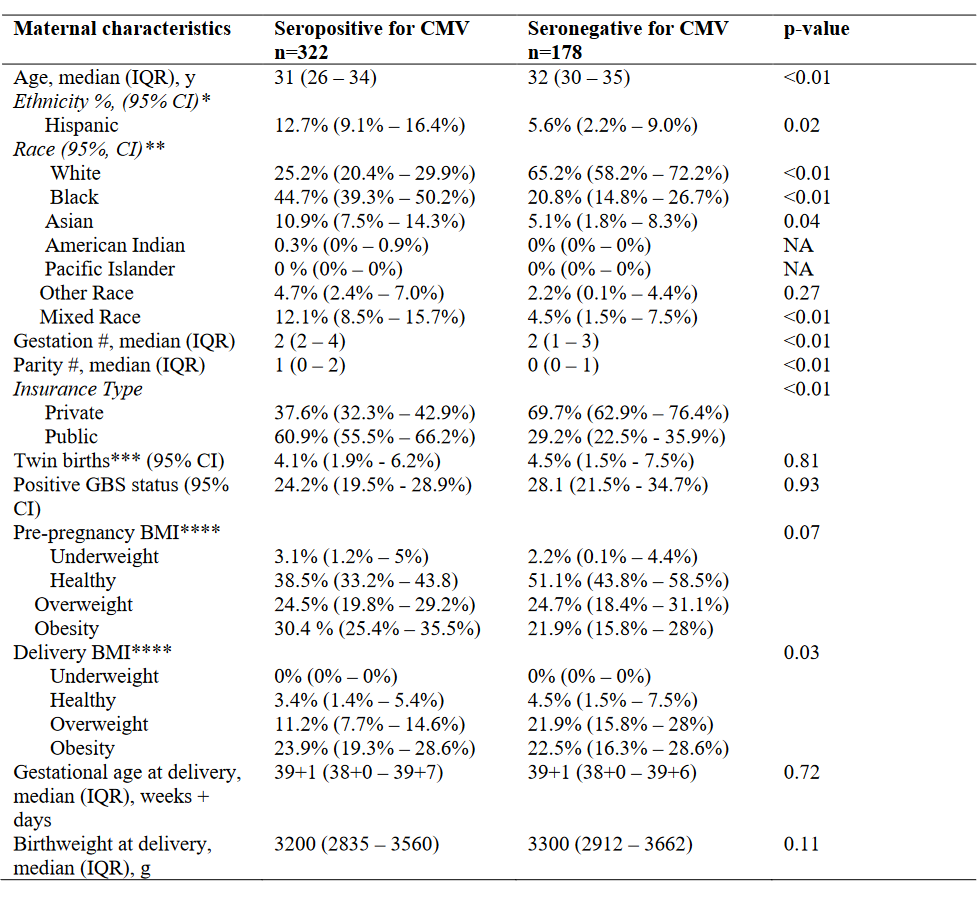 * From the available 500 records, 414 had ethnicity registered. ** From the available 500 records, 489 had race registered. ****Definition per CDC categories.
* From the available 500 records, 414 had ethnicity registered. ** From the available 500 records, 489 had race registered. ****Definition per CDC categories.
