Asthma 2
Session: Asthma 2
478 - Feasibility and acceptability of using a home, digital indoor air quality monitor among children with asthma
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 478.4620
Kristin Kan, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Olivia Orr, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Kyle S. Honegger, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Lizbeth Sanchez, DePaul University, Burbank, IL, United States; Kiran Bhat, Northwestern University, Evanston, IL, United States; Sai Nimmagadda, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States

Kristin Kan, MD MPH MSc (she/her/hers)
Assistant Professor
Ann & Robert H. Lurie Children's Hospital of Chicago
Chicago, Illinois, United States
Presenting Author(s)
Background: With children spending over 80% of their time indoors, poor indoor air quality can be a primary source of triggers for adverse asthma symptoms. Digital air sensors can monitor and measure indoor air; but there is not widespread use in non-commercial, indoor air quality monitoring.
Objective: To assess the feasibility and acceptability of providing families with children with asthma with a digital, in-home air quality monitor for 3 months.
Design/Methods: Twenty caregiver-child pairs were recruited from December 2023 to February 2024 from a pediatric pulmonary practice in an urban setting. Children had physician-diagnosed persistent asthma. Caregivers were given a digital in-home air quality sensor to place in their child's bedroom for 3 months that transmitted air quality data to a dashboard for the research team. Mobile Wi-Fi hotspots were provided to families to remove any internet access barriers. We collected continuous indoor air quality data, demographic information, asthma-related measures (asthma control and self-efficacy in pediatric asthma), and usability from the System Usability Score (SUS). We conducted a brief exit interview for assessing feasibility and acceptability of the indoor air quality device.
Results: Caregivers were primarily female and parents of the child (Table 1). Children's Asthma Control Test scores were 19 [IQR: 18, 21]. Nineteen of 20 enrolled participants collected indoor air data. On average, participants provided data for 81 days, with data collected 72% of the available hours (min: 34%, max: 97%). Data was collected for at least 12 hours on an average of 74% of days. Indoor air quality measures for carbon monoxide, nitrogen dioxide, and particulate matter 2.5 were above the World Health Organization suggested standards. (Table 2) For acceptability, all caregivers (100%, n=15) found the air quality sensor easy or very easy to set-up; and 87% ( n=13) reported that they were likely or very likely to use the air quality sensor in the future, if provided in clinic, and to recommend this technology to another family. Caregivers described indoor air monitoring as "how to do better and how to improve our chances at healthy living for our kids, and maybe even for ourselves.” (Table 3) Caregivers scored 67.5 [IQR: 56.3, 81.2] on the SUS.
Conclusion(s): In general, using digital indoor air sensors was feasible and acceptable for monitoring air quality in the homes of caregivers of children with asthma. Future studies should explore how to integrate this digital tool with other asthma management supports for improving indoor air for children with asthma at home.
Table 1. Characteristics of Study Sample (n= 20)
.png)
Table 2. Summary of Indoor Air Quality Metric Averages (n = 19)
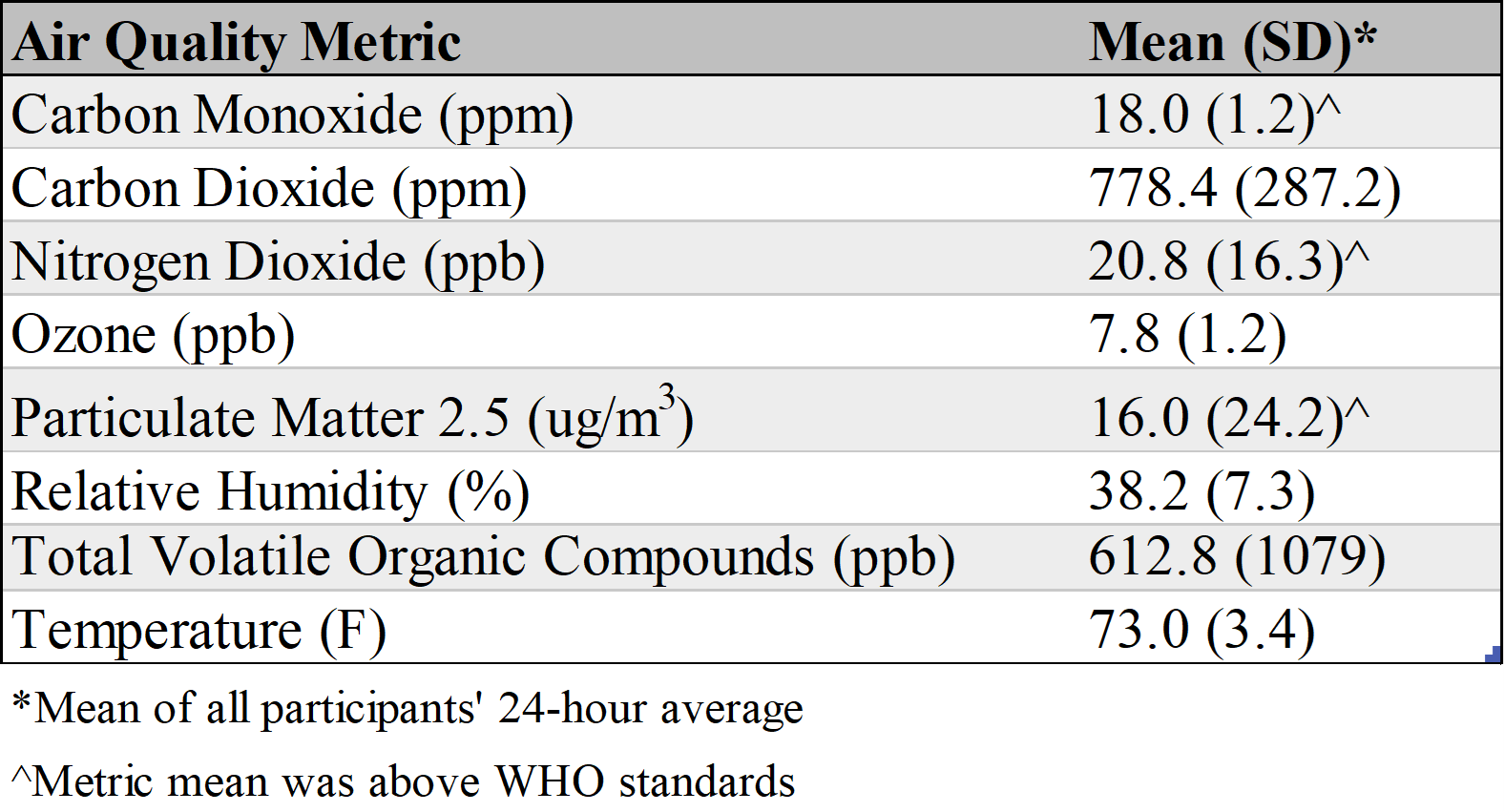
Table 3. System Usability Scale and Acceptability Interview Responses
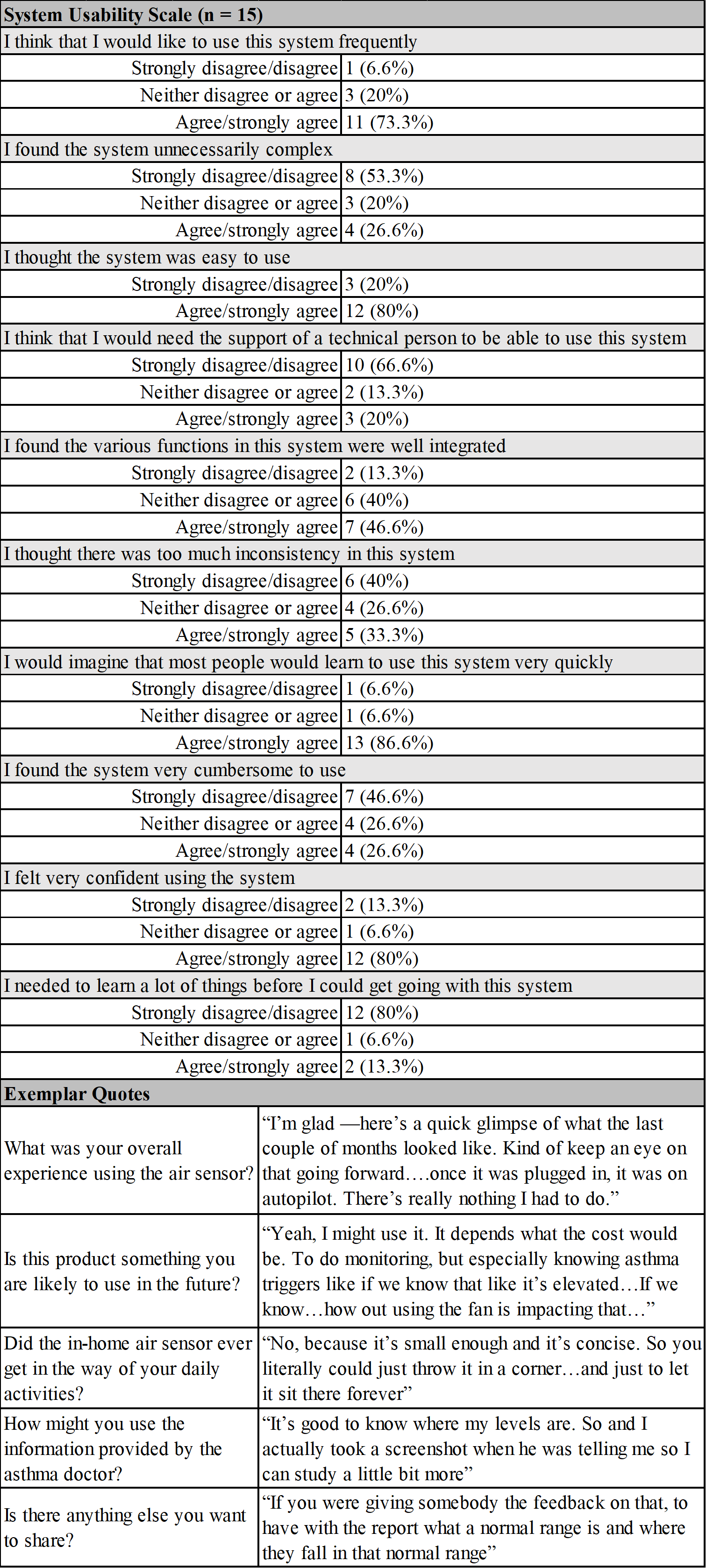
Table 1. Characteristics of Study Sample (n= 20)
.png)
Table 2. Summary of Indoor Air Quality Metric Averages (n = 19)

Table 3. System Usability Scale and Acceptability Interview Responses


